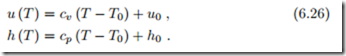Monatomic Gases (Noble Gases)
For monatomic gases, i.e., the noble gases helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn), the specific heats are true constants with the values
and the caloric equation of state follows from straightforward integration as
With cp = const, the integration in (6.20) can be performed easily, and the entropy becomes
Since the resulting expressions for the thermodynamic quantities of monatomic gases are rather simple, these are typically not tabulated.
Related posts:
Centrifugal Pumps, Fans, and Compressors:Head increase of a centrifugal pump.
The Chemical Potential:Properties of the Chemical Potential.
Other Automatic Controls:High-Pressure Cutout Switch
Real Gases:Ideal Gas
Furnace Fundamentals:Horizontal Furnace
Example: Safety Valve
Closed System Cycles:Dual Cycle and Atkinson Cycle
Steam Heating Systems:Steam Boilers
Heat Pumps:Other Types of Heat Pumps and Dual-Fuel Heat Pump System