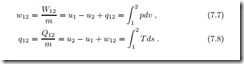Standard Processes
In Chapter 8 we shall study thermodynamic cycles in closed systems which model thermal engines, including internal combustion engines. The focus will lie on the understanding of the working principles of the cycles, and on the main parameters that determine their efficiency. For this it is customary to base the analysis on reversible processes, which allow a full analysis.
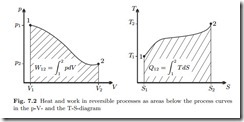
There are a number of processes that are often realized (at least approximately) in thermodynamic systems: processes at constant volume, constant pressure, constant temperature, or adiabatic processes. Typical thermodynamic cycles consist of closed chains of several of these processes. In this chapter we compute work and heat for these standard processes as a reference for the discussion of cycles.
Basic Equations
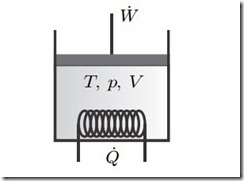
Figure 7.1 shows, again, a piston-cylinder device as the prototypical closed system. In reversible (quasi-static) processes, the system exchanges energy
Fig. 7.1 Closed system with piston work and heat exchange. In this chapter we are interested in reversible processes only, so there is not stirring.
through heating and piston work only; stirring (propeller work) as an irreversible process is excluded. All movement of the material in the system is so slow that velocity and kinetic energy can be ignored. For a stationary system, potential energy is constant and can be ignored as well. Thus, at all times the system is in homogeneous equilibrium states which are characterized by the temperature T , the pressure p, and the volume V .
We list the relevant equations from previous chapters. Under the above simplifications, the first law for closed systems reduces to
where Q˙ is the heat transfer rate, and W˙ denotes power. Integration over the duration of the process gives the time-integrated energy balance
are the total amounts of heat and work exchanged between the states 1 (at time t1) and 2 (at time t2).
For an infinitesimal step of the process (duration dt) we have the differential form of the first law
In the following sections we shall compute work and heat per unit mass, which for reversible processes are given by