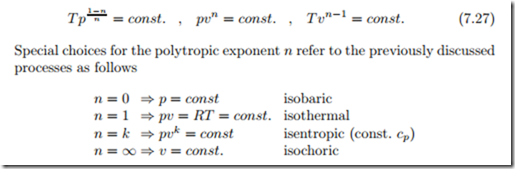
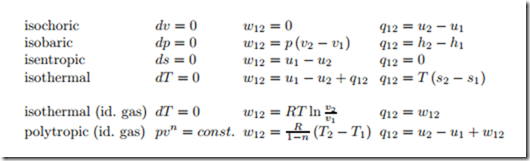Polytropic Process (Ideal Gas): pvn = const
Processes in actual applications might differ from those discussed above. A useful approximate description of a wide variety of processes in ideal gases is offered by the polytropic process, which is a generalization of the adiabatic relations (7.23) to arbitrary exponents n,
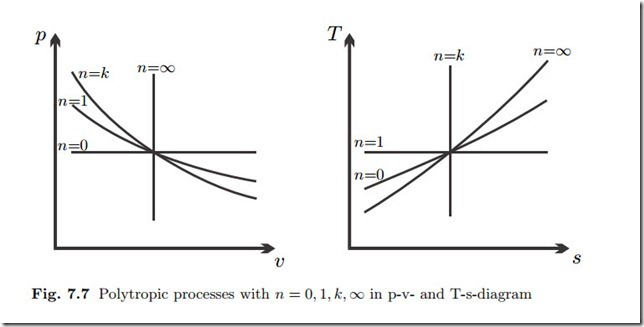
Often one uses values of n in the interval 1 ≤ n ≤ k to describe processes that are not fully adiabatic and not fully isothermal, e.g., compression or expansion processes with small heat exchange. Figure 7.7 shows the various processes in the two diagrams.
which holds for all n /= 1. The work for the case n = 1 (isothermal) can be found from the above by using l’Hˆopital’s rule:
Summary
For easy reference, we collect the results of this section in a table,