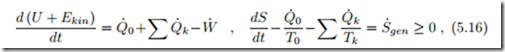Internally and Externally Reversible Processes
For a sound thermodynamic evaluation of processes it is important to identify and understand all causes for work loss to irreversible processes. Even if a process is reversible within the boundaries of the system considered, there might be associated irreversible processes outside the system boundaries. For the thorough evaluation of the performance of a system, in particular for accounting for the associated work losses inside and outside the system, the following definitions are useful:
Internally reversible process: No irreversible processes occur inside the system boundaries.
Externally reversible process: No irreversibilities occur outside the sys- tem boundaries.
Fully reversible process: A process which is both, externally and internally reversible.
Irreversibility and Work Loss
The thermodynamic laws for closed systems that exchange heat with an arbitrary number of reservoirs read
where the heat exchange Q˙ 0 with a reservoir at T0 is highlighted. Most thermodynamic engines utilize the environment as heat source or sink, and in this case Q˙ 0 should be considered as the heat exchanged with the environment.
Note that the environment is freely available, and no cost is associated with removing heat from, or rejecting heat into, the environment. For the heat engines of Sec. 5.2 and the heat pumps of Sec. 5.4 the environmental temperature is T0 = TL, while for the refrigerators of Sec. 5.4 we have T0 = TH .
Elimination of Q˙ 0 between the two laws and solving for work gives
This equation generalizes the findings of the previous sections to arbitrary processes in closed systems: The generation of entropy in irreversible processes reduces the work output of work producing devices (where W˙ > 0, e.g., heat engines) and increases the work requirement of work consuming devices (where W˙ < 0, e.g., heat pumps and refrigerators). We note the appearance of the Carnot factor / Tk multiplying the heating rates Qk .
The amount of work lost to irreversible processes is
![]() sometimes it is denoted as the irreversibility. It is an important engineering task to identify and quantify the irreversible work losses, and to reduce them by redesigning the system, or use of alternative processes.
sometimes it is denoted as the irreversibility. It is an important engineering task to identify and quantify the irreversible work losses, and to reduce them by redesigning the system, or use of alternative processes.