REFRIGERANTS: NEW AND OLD
Refrigerants are used in the process of refrigeration, which is a process whereby heat is removed from a sub- stance or a space. A refrigerant is a substance that picks up latent heat when it evaporates from a liquid to a gas. This is done at a low temperature and pressure. A refrigerant expels latent heat when it condenses from a gas to a liquid at a high pressure and temperature. The refrigerant cools by absorbing heat in one place and discharging it in another area.
The desirable properties of a good refrigerant for commercial use are:
• Low boiling point
• Safe, nontoxic
• Easy to liquefy and moderate pressure and temperature
• High latent heat value
• Operation on a positive pressure
• Not affected by moisture
• Mixes well with oil
• Noncorrosive to metal
There are other qualities that all refrigerants have: molecular weight, density, compression ratio, heat value, and compression temperature. These qualities will vary with the refrigerant used. The compressor displacement and type or design will also influence the choice of refrigerant.
CLASSIFICATION
Refrigerants are classified according to their manner of absorption or extraction of heat from substances to be refrigerated:
• Class 1 refrigerants are used in the standard compression type of refrigeration systems
• Class 2 refrigerants are used as immediate cooling agents between Class 1 and Class 3 types and the substance to be refrigerated
• Class 3 refrigerants are used in the standard absorption type of refrigerating systems
Class 1 refrigerants cool by absorption or extraction of latent heat from the substances.
Class 2 refrigerants cool substances by absorbing their sensible heats. They include air, calcium-chloride brine, sodium-chloride (salt) brine, alcohol, and similar non-freezing solutions.
Class 3 refrigerants consist of solutions that contain absorbed vapors of liquefiable agents or refrigerating media. These solutions function through their ability to carry the liquefiable vapors. The vapors produce a cooling effect by the absorption of latent heat. An example is aqua ammonia, which is a solution composed of distilled water and pure ammonia.
COMMON REFRIGERANTS
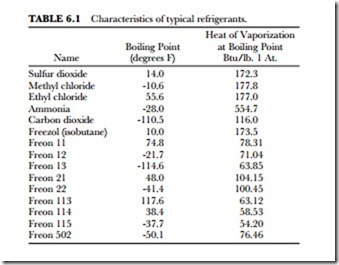
Following are some of the more common refrigerants. Table 6.1 summarizes the characteristics to a selected few of the many refrigerants available for home, commercial and industrial use.
Sulfur dioxide (SO2) is a colorless gas or liquid. It is toxic, with a very pungent odor. When sulfur is burned in air, sulfur dioxide is formed. When sulfur dioxide combines with water, it produces sulfuric and sulfurous acids. These acids are very corrosive to metal. They have an adverse effect on most materials. Sulfur dioxide is not considered a safe refrigerant. As a refrigerant, sulfur dioxide operates on a vacuum to give the temperatures required. Moisture in the air will be drawn into the system when a leak occurs. This means the metal parts will eventually corrode, causing the compressor to seize.
Sulfur dioxide (SO2) boils at 14 degrees F (-10 degrees C) and has a heat of vaporization at boiling point (1 atmosphere) of 172.3 Btu/pound. It has a latent heat value of 166 Btu per pound.
To produce the same amount of refrigeration, sulfur dioxide requires about one-third more vapor than Freon® and methyl chloride. This means that the condensing unit has to operate at a higher speed or that the compressor cylinders must be larger. Since sulfur dioxide does not mix well with oil, the suction line must be on a steady slant to the machine. Otherwise the oil will trap out, constricting the suction line. This refrigerant is not feasible for use in some locations.
Methyl Chloride
Methyl chloride (CHP) has a boiling point of -10.6 degrees F (-23.3 degrees C). Its heat of vaporization at boiling point (at l atmosphere) is 177.8 Btu/pound. It is a good refrigerant. However, because it will burn under some conditions, some cities will not allow it to be used. It is easy to liquefy and has a comparatively high latent heat value. It does not corrode metal when in its dry state.
However in the presence of moisture it damages the compressor. A sticky black sludge is formed when ex- cess moisture combines with the chemical. Methyl chloride mixes well with oil. It will operate on a positive pressure as low as -10 degrees F (-23 degrees C). The amount of vapor needed to cause discomfort in a per- son is in proportion to the following numbers:
That means methyl chloride is 35 times safer than ammonia and 70 times safer than sulfur dioxide. Methyl chloride is hard to detect with the nose or eyes. It does not produce irritating effects. Therefore,
some manufacturers add a 1 percent amount of acrolein, a colorless liquid with a pungent odor, as a warning agent. It is produced by destructive distillation of fats.
Ammonia
Ammonia (NH3) is used most frequently in large industrial plants. Freezers for packing houses usually em- ploy ammonia as a refrigerant. It is a gas with a very noticeable odor. Even a small leak can be detected with the nose. Its boiling point at normal atmospheric pressure is -28 degrees F (-33 degrees C). Its freezing point is -107.86 degrees F (-77.7 degrees C). It is very soluble in water. Large refrigeration capacity is possible with small machines. It has high latent heat (555 Btu at 18 degrees F (-7.7 degrees C). It can be used with steel fittings. Water-cooled units are commonly used to cool down the refrigerant. High pressures are used in the lines (125 to 200 pounds per square inch). Anyone inside the refrigeration unit when it springs a leak is rap- idly overcome by the fumes. Fresh air is necessary to reduce the toxic effects of ammonia fumes. Ammonia is combustible when combined with certain amounts of air (about one volume of ammonia to two volumes of air). It is even more combustible when combined with oxygen. It is very toxic. Heavy steel fittings are required since pressures of 125 to 200 pounds per square inch are common. The units must be water-cooled.
Carbon Dioxide
Carbon dioxide (CO2) is a colorless gas at ordinary temperatures. It has a slight odor and an acid taste. Carbon dioxide is non-explosive and non-flammable. It has a boiling point of 5 degrees F (-15 degrees C). A pressure of over 300 pounds per square inch is required to keep it from evaporation. To liquefy the gas, a condenser temperature of 80 degrees F (26.6 degrees C) and a pressure of approximately 1000 pounds per square inch are needed. Its critical temperature is 87.8 degrees F (31 degrees C). It is harmless to breathe except in extremely large concentrations. The lack of oxygen can cause suffocation under certain conditions of carbon-dioxide concentration.
Carbon dioxide is used aboard ships and in industrial installations. It is not used in household applica- tions. The main advantage of using carbon dioxide for a refrigerant is that a small compressor can be used. The compressor is very small since a high pressure is required for the refrigerant. Carbon dioxide is, how- ever, very inefficient compared to other refrigerants.
Calcium Chloride
Calcium chloride (CaCl2) is used only in commercial refrigeration plants. It is used as a simple carrying medium for refrigeration.
Brine systems are used in large installations where there is danger of leakage. They are also used where the temperature fluctuates in the space to be refrigerated. Brine is cooled down by the direct expansion of the refrigerant. It is then pumped through the material or space to be cooled. Here it absorbs sensible heat.
Most modern plants operate with the brine at low temperature. This permits the use of less brine, less pip- ing or smaller-diameter pipe, and smaller pumps. It also lowers pumping costs. Instead of’ cooling a large volume of brine to a given temperature, the same number of refrigeration units are used to cool a smaller volume of brine to a lower temperature. This results in greater economy. The use of extremely low-freezing brine, such as calcium chloride, is desirable in the case of the shell-type cooler. Salt brine with a minimum possible freezing point of -6 degrees F (-20.9 degrees C) may solidify under excess vacuum on the cold side of the refrigerating unit. This can cause considerable damage and loss of operating time. There are some cases in which the cooler has been ruined.
Ethyl Chloride
Ethyl chloride (C2H5Cl) is not commonly used in domestic refrigeration units. It is similar to methyl chlo- ride in many ways. It has a boiling point of 55.6 degrees F (13.1 degrees C) at atmospheric pressure. Criti- cal temperature is 360.5 degrees F (182.5 degrees C) at a pressure of 784 pounds absolute. It is a colorless
liquid or gas with a pungent ethereal odor and a sweetish taste. It is neutral toward all metals. This means that iron, copper, and even tin and lead can be used in the construction of the refrigeration unit. It does, however, soften all rubber compounds and gasket material. Thus, it is best to use only lead for gaskets.
Freon® Refrigerants
Freon® refrigerants have been one of the major factors responsible for the tremendous growth of the home refrigeration and air conditioning industries. The safe properties of these products have permitted their use under conditions where flammable or more toxic refrigerants would be hazardous to use. There is a Freon refrigerant for every application—from home and industrial air conditioning to special low-temperature requirements.
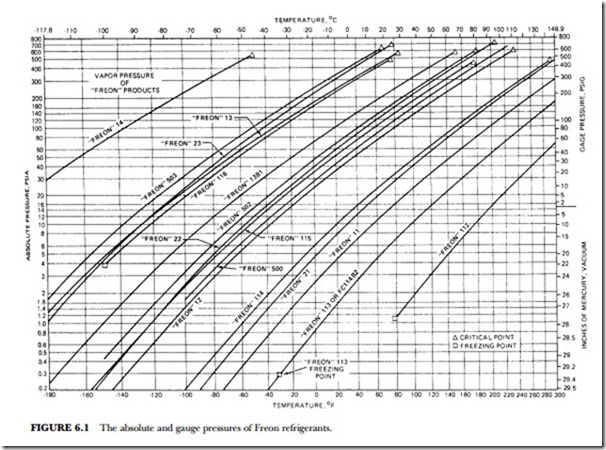
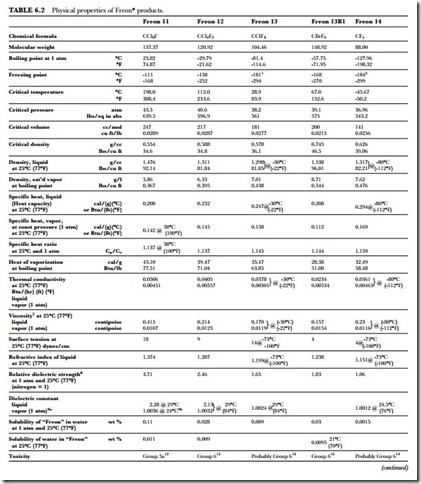
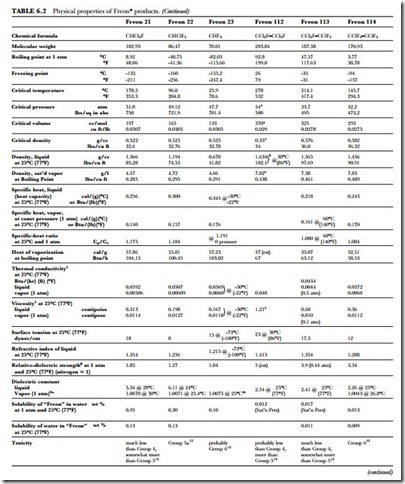
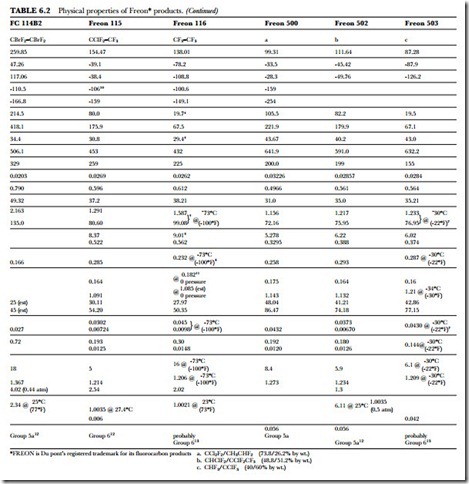
The unusual combination of properties found in the Freon compounds is the basis for their wide application and usefulness. Table 6.2 presents a summary of the specific properties of some of the fluorinated products. Figure 6.1 gives the absolute pressure and gauge pressure of Freon refrigerants at various temperatures.