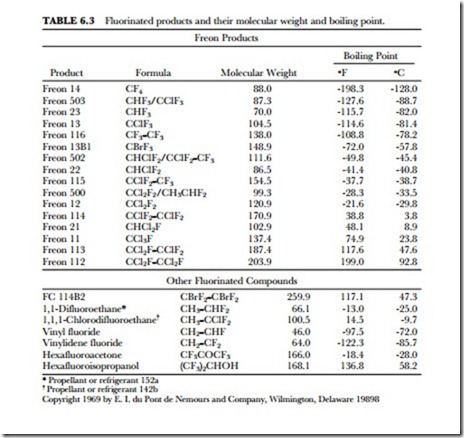
MOLECULAR WEIGHTS
Compounds containing fluorine in place of hydrogen have higher molecular weights and often have unusually low boiling points. For example, methane (CH4), with a molecular weight of 16, has a boiling point of –degrees F (-161.4 degrees C) and is nonflammable. Freon® 14 (CF4) has a molecular weight of 88 and a boiling point of -198.4 degrees F (-128 degrees C) and is nonflammable. The effect is even more pronounced when chlorine is also present. Methylene chloride (CH2Cl2) has a molecular weight of 85 and boils at 105.2 degrees F (40.7 degrees C), while Freon 12 (CCl2F2, molecular weight 121) boils at -21.6 degrees F (-29.8 degrees C). It can be seen that Freon compounds are high-density materials with low boiling points, low viscosity, and low surface tension. Freon products have boiling points covering a wide range of temperatures. (See Table 6.3.)
The high molecular weight of the Freon compounds also contributes to low vapor, specific heat values, and fairly low latent heats of vaporization. Tables of thermodynamic properties including enthalpy, entropy, pressure, density, and volume for the liquid and vapor are available from manufacturers.
Freon compounds are poor conductors of electricity. In general, they have good dielectric properties.
FLAMMABILITY
None of the Freon compounds are flammable or explosive. However, mixtures with flammable liquids or gases may be flammable and should be handled with caution. Partially halogenated compounds may also be flammable and must be individually examined.
TOXICITY
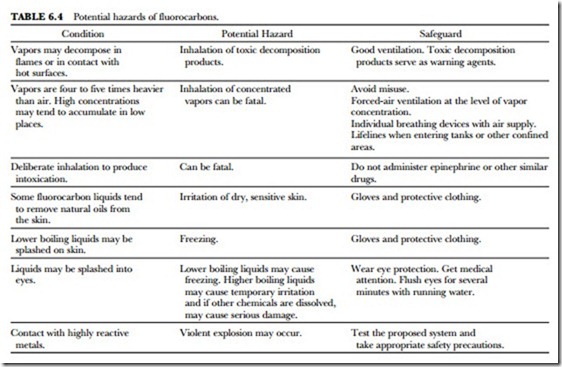
One of the most important qualities of the Freon fluorocarbon compounds is their low toxicity under nor- mal conditions of handling and usage. However, the possibility of serious injury or death exists under un- usual or uncontrolled exposures or in deliberate abuse by inhalation of concentrated vapors. The potential hazards of fluorocarbons are summarized in Table 6.4.
SKIN EFFECTS
Liquid fluorocarbons with boiling points below 32 degrees F (0 degrees C) may freeze the skin, causing frost- bite on contact. Suitable protective gloves and clothing give insulation protection. Eye protection should be used. In the event of frostbite, warm the affected area quickly to body temperature. Eyes should be flushed
copiously with water. Hands may be held under armpits or immersed in warm water. Get medical attention immediately. Fluorocarbons with boiling points at or above ambient temperature tend to dissolve protective fat from the skin. This leads to skin dryness and irritation, particularly after prolonged or repeated contact. Such contact should be avoided by using rubber or plastic gloves. Eye protection and face shields should be used if splashing is possible. If irritation occurs following contact, seek medical attention.
ORAL TOXICITY
Fluorocarbons are low in oral toxicity as judged by single-dose administration or repeated dosing over long periods. However, direct contact of liquid fluorocarbons with lung tissue can result in chemical pneumonitis, pulmonary edema, and hemorrhage. Fluorocarbons 11 and 113, like many petroleum distillates, are fat solvents and can produce such an effect. If products containing these fluorocarbons were accidentally or purposely ingested, induction of vomiting would be contraindicated.