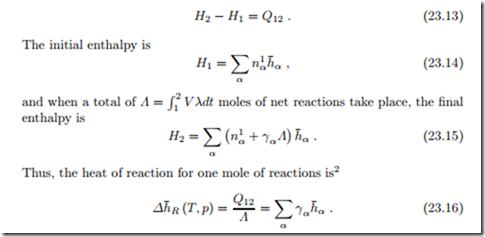Heat of Reaction
Chemical reactions are accompanied by changes in energy. We consider a mixture in a piston-cylinder system at (T, p), in which reactions take place at constant pressure and temperature. The heat of reaction is defined as the amount of heat that must be exchanged to keep temperature and pressure constant.
Since the system is isobaric, the first law assumes the form
A reaction with Δh¯R < 0 is an exothermic reaction, heat must be with- drawn to keep the temperature constant. A reaction with Δh¯R > 0 is an endothermic reaction, heat must be added to keep the temperature constant.
The heat of reaction can be measured. Normally, one finds Δh¯R tabulated at standard conditions, i.e. at T0 = 298.15 K, p0 = 1 bar. Some values are given in the following table:
If one of the products is water, the heat of reaction depends on whether the product water is liquid (l) or vapor (v). Note that a change of sign or value of the stoichiometric coefficients changes sign and value of the heat of reaction; e.g., for the reaction 2H2O (l) 2H2 + 1O2, we find Δh¯R = 571.6 kJ .