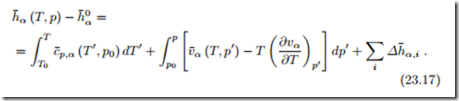Heating Value
The heating value is defined as the energy released in the combustion of 1 kg of fuel at reference conditions. One distinguishes between the lower heating value (LHV), where the product water is vapor (v) and the higher heating value (HHV), where the product water is liquid (l). The difference is just the heat of evaporation of the the product water at standard reference temperature T0 which is 2442 kJ or 43.96 kJ .
For instance for the combustion of methane (CH4), the higher and the lower heating values are
Enthalpy of Formation
When we discussed the measurement of properties, it became clear that internal energy, enthalpy and entropy cannot be measured directly. What can be measured is, for instance, the specific heat at constant pressure, and the thermal equation of state. The enthalpy follows by integration, as shown in (16.34), which for the molar enthalpy of α assumes the form
When chemical reactions take place, the reference enthalpy ¯0 =h¯α (T0, p0) cannot be chosen arbitrarily. Indeed, for a reaction occurring at (T0, p0) the heat of reaction—which can be measured—is Δh¯R (T0, p0) = >: γαh¯0 . Thus, the reference enthalpies ¯h0 for different substances must be properly related, so that they give the proper, i.e., measured, heat of reaction—for all possible reactions.
To ensure this, the following convention is used to define the reference enthalpies as enthalpies of formation h¯0 at (T0, p0): Stable elements at (T0, p0), such as O2, N2, H2, C are assigned values of zero enthalpy,
While it would be convenient to use the h¯0 as reference for thermodynamic property tables, this is most often not done. Most property tables that list enthalpy and internal energy as functions of temperature and pressure refer to other reference states. For ideal gas tables it is customary to scale such that the extrapolated enthalpy at T = 0 K is zero. Vapor tables often have the internal energy or the enthalpy at the triple point set to zero. Thus, the tabulated values must be re-scaled before they are used for computations involving chemical reactions.
We discuss how the proper data can be found, when the molar enthalpy h˜α (T, p) is tabulated. When no chemical reactions occur, the tabulated data can be used as is. However, when chemical reactions are considered, the tabulated values h˜α (T, p) must be corrected to the proper reference. Since enthalpy differences are independent of the choice of the reference state, and since the enthalpy of formation is the enthalpy at (T0, p0), we have
Here, h˜α (T, p) denotes tabulated enthalpy values, and h¯α (T, p) denotes the enthalpies with proper reference value that must be used for the discussion of chemical reactions.
Water is a common reaction product, in particular when combustion of fuels is considered. Pure water at (T0, p0) is liquid, with −285.83 kJ , but when the product is a gas mixture some or all of the water can be in the vapor state, with h¯0 psychrometrics for more discussion.