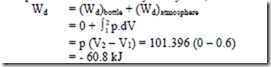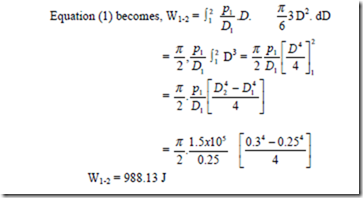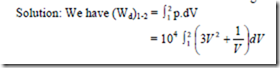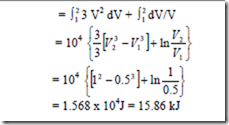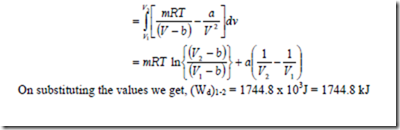Problems:
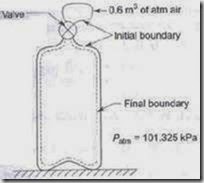
1. Gas from a bottle of compressed helium is used to inflate a balloon originally folded completely flat, to a volume of 0.25 m3. If the barometer reads 760 mm of mercury, how much work is done by the system comprising the helium initially in the bottle, if the balloon is light and requires no stretching. Sketch the system before and after the process.
Solution:
The firm line S1 shows the boundary of the system before the process, and the dotted line S2 shows the boundary after the process.
Total displacement work is given by
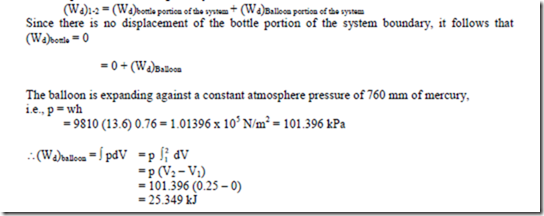
2. Determine the work done by the air which enters an evacuated bottle from the atmosphere when the cork is opened, atmospheric air rushes into it. If the atmospheric pressure is
101.396 kPa and 0.6m3 of air (measured at atmosphere conditions) enters the bottle. Solution:
No work is done by the part of the boundary in contact with the bottle. Work is done only by the moving part external to the bottle. The pressure over this moving part is uniform at 101.396 kPa
Displacement work done by the system,
Negative, because the boundary is contracting. Thus the surroundings do positive work at the boundary and the work done by the air negative
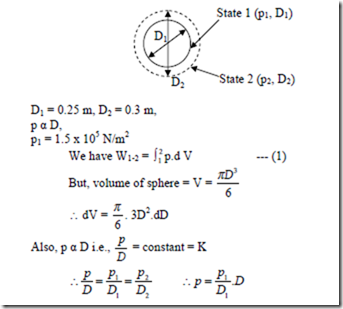
3. A spherical balloon has a diameter of 25 cm and contains air at a pressure of 1.5 x 105Pa. The diameter of the balloon increases to 30 cm in a certain process and during this process the pressure is proportional to the diameter. Calculate the work done by the air inside the balloon during this process.
Solution:
Positive sign indicates that work is done by the system.
4. Gas from a bottle of compressed helium is used to inflate an inelastic flexible balloon, originally folded completely flat to a volume of 0.5 m3. If the barometer reads 760 mm of Hg, what is the amount of work done upon the atmosphere by the balloon (50.66 kJ)
5. When the valve of the evacuated bottle is opened, atmosphere air rushes into it. If the atmosphere pressure is 101.325 KPa, and 1.2 m3 of air (measured at atmosphere conditions) enters the bottle, calculate the work done by the air (-60.8 kJ).
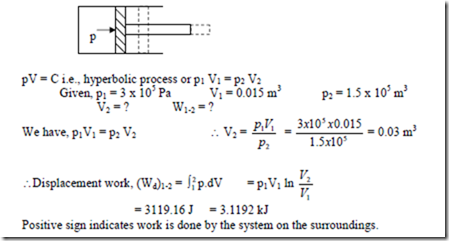
6. A gas system, confined by a piston and cylinder, undergoes a change of state such that the product of pressure and volume remains constant. If the process begins at a pressure of 3 bar and a volume 0.015m3 and proceeds until the pressure falls to half its initial value, determine the magnitude and direction of the work flow.
Solution:
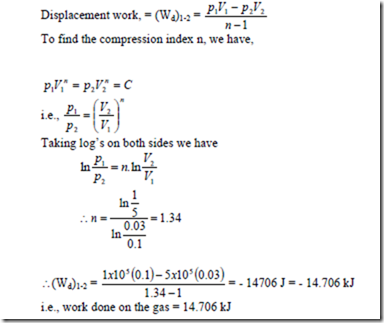
7. A certain amount of gas is compressed from 1 bar and 0.1m3 to 5 bar and 0.03m3. The process is according to the law pVn = K. Determine the magnitude and direction of work.
Solution: Given: p1 = 1 bar; V1 = 0.1 m3; p2 = 5 bar; V2 = 0.03 We have for a polytropic process,
8. A gas confined in a cylinder by a piston is at pressure of 3 bar and a volume of 0.015 m3. The final pressure is 1.5 bar. Determine the magnitude and direction of work transfer for the
-ve sign indicates that work is done on the system
9. A non-flow reversible process occurs for which p = 3V2 + 1/V where p is in N/cm2 and V is in m3. What is the work done when V changes from 0.5 m2 to 1 m3.
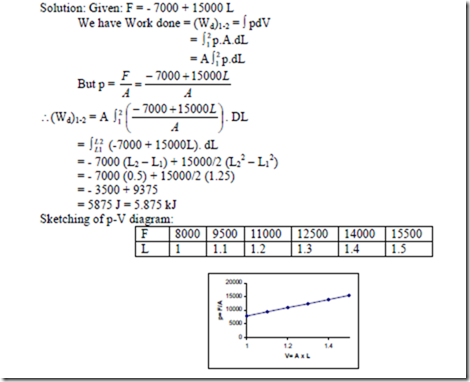
10. A system consists of a cylinder and piston machine. The external normal load applied to the piston is given by F = – 7000 + 15000L Newton’s, where L is the distance in mts from the closed and of the cylinder to the piston. How much work is done when the piston moves from the position L = 1m to L = 1.5 m. Sketch the p-V diagram for this process and show the work done.
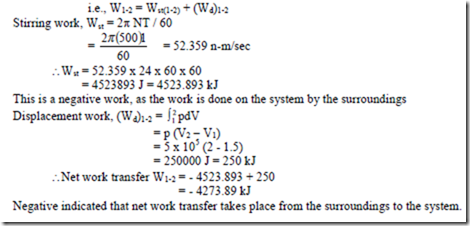
11. An insulated system contains a mixture of ice and water. A paddle wheel is rotated in the system at 100rpm. Torque applied to the shaft is 3 N-m. In order to effect the transformation of 1 kg of ice to liquid water 300 kJ of heat must be transferred to the system. Determine the length of time the paddle wheel must be rotated in order to achieve 2.5 kg reduction in the quantity of ice.
Solution: Given: T = 3 N-m ; N = 1000 rpm
12. A system containing 5 kg of a substance is stirred with a torque of 1 N-m at a speed of 500 rpm for 24 hrs. The system mean while expands from 1.5m3 to 2.0m3 against a constant pressure of 5 bar. Determine the magnitude and direction of net work transfer.
Solution: The system is associated with two interactions with the surroundings i.e., stirring work (surroundings to the system) and displacement work
13. A mass of 1.5 kg of a substance is compressed in a quasi-static process from 0.1 MPa to 0.7 MPa. The initial pressure density of the substance is 1.16 kg/m3. Determine the magnitude of work done on the substance if i) process is pV = C and pV1-4 = C
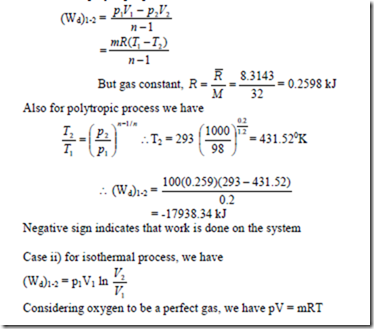
14. O2 is compressed in a quasi static process according to the relation pV1-2 = C. The initial conditions are 98 KPa and 200 C and the final pressure is 1000 KPa. Assuming an ideal gas behaviour, determine the work required to compress 100 kg of O2. Compare this work with the work of isothermal compression, i.e., pV = C.
Solution: p1 =98 x 103 Pa, T1 = 2930 K, p2 = 1000 x 103Pa, m = 100 kg We have for polytropic process,
15. The following data refer to a12 Cylinder, single-acting, two-stroke marine Diesel engine: Cylinder diameter-0.8m
Stroke of piston-1.2m
Area of indicator diagram-5.5E10-4 m2
Length of diagram-0.06m
Spring value-147 MPa per m
Find the net rate of work transfer from the gas to the piston in kW.
Solution: Mean effective pressure, Pm, is given by
 One engine cycle is completed in two strokes of the piston or one revolution of the crank shaft.
One engine cycle is completed in two strokes of the piston or one revolution of the crank shaft.
Work done in one minute= Pm LAN
Since the engine is single-acting, and it has 12 cylinders, each contributing an equal power, the rate of work transfer from the gas to the piston is given by
16. A gas system has mass m, occupies a volume V at a pressure of p and temperature T. These properties are related by the equation ![]() where a, b and R are constants. Obtain an expression for the displacement work done by this gas system during a constant temperature process where the gas expands from 1 m3 to 10 m3 at a temperature of 293 K. Assume a = 15.7 x 104 Nm4, b = 1.07 x 10-2 and R = 0.278kJ/kg-K.
where a, b and R are constants. Obtain an expression for the displacement work done by this gas system during a constant temperature process where the gas expands from 1 m3 to 10 m3 at a temperature of 293 K. Assume a = 15.7 x 104 Nm4, b = 1.07 x 10-2 and R = 0.278kJ/kg-K.
Related posts:
Incoming search terms:
- A spherical ballon has a diameter of 0 3m and contains air at a pressure of 1 5bar calculate the workdone
- thermo time to inflate balloon problem
- A gas system compressed by a piston and cylinder undergoes a change of state such that the product of pressure and volume remains constant If the process begins at a pressure of 300 KPa and a volume of 0 015 m3 and proceeds until the pressure falls to hal
- The volume of a gas expands by 0 25m3 at a constant pressure of 10to the power 3 N m2 the work done is equal to
- gas from bottle of compressed heliun is used to in flaked
- a spherical balloon of 1m diameter contains a gas of 150kpa the gas inside the balloon is heated until pressure reaches 450 kpa
- a spherical baloon of 1m diameter contains a gas at 200 kpa
- a piston machine contains air at apressure of 12 bar which occupies a volume of 0 02mcube
- A mass of 1 5 kg of air is compressed in a quasi-static process from 0 1 MPa to 0 7 MPa for which pv constant The initial density of air is 1 16 kg/m Find the work done by the piston to compress the air
- A spherical balloon of 1m diameter contains a gas at 200kPa The gas inside the balloon is heated until the pressure reaches 500kPa During the process of heating the pressure of gas inside the balloon is proportional to the diameter of the balloon Make cal
- afluid at0 7bar occupying 0 9m3 is compressed reversibly to3 5bar according to alaw
- work done by air to fill an evacuated bottle?
- A spherical balloon of 1 m diameter contains a gas at 150 kPa The gas inside balloon is heated until pressure reaches 450 kPa During the process of heating the pressure of gas inside the balloon is proportional to cube of the diameter of the balloon Find
- a cylinder contains 5m cube of ideal gas at 1bar this gas is copressed in quasi equilibirium isothermal process till its pressure rises to 5bar find work required
- air is injected from cylinder of compressed air into a ballon of volume v causing its radius to double what is the work done against pressure p of atmosphere
- a spherical balloon of 1 m diameter contains a gas at 150 kpa
- workdome in ballon heatimg ehen pressure directly proportional to square of dia
- a spherical ballon of 1m dia contains a gas of 150kpa solution
- a spherical balloon contains 5kg of air at 200 kpa
- a spherical balloon contains air at 150 kPa
- a spherical balloon contains air at temperature T0 and pressure P0
- a spherical balloon of 1m dia contains gas
- a spherical balloon of 1 meter diameter contains a gas of 0 15N/mm square the gas inside the balloon is heated until pressure reaches 0 45N/mm square during the process the pressure of gas is proportional to Cube of diameter of the balloon find the workdo
- a spherical balloon has diameter of 25cm
- a spherical balloon has a diameter of 25 cm and contain air at pressure of 150kpa the diameter of the balloon increases to 30 cm in certain process during this process the pressure of gas inside the balloon is proportional to diameter of the balloon what
- a spherical balloon of 1 m diameter contains solution
- a spherical balloon of 1 m diameter
- a spherical balloon of 1 m diameter contains a gas at 150 kpa The gas inside balloon is heated until pressure reaches 450kpa
- A spherical balloon of 1m diameter contains a gas at 150 kPa the gas inside balloon is heated until pressure reaches 450kPa During the process of heating the pressure of gas inside the balloon is proportional to cube of the diameter of balloon find the wo
- A spherical balloon of 1 m diameter contains a gas at 0 15N/mm^2 the gas inside the balloon is heated until the pressure reaches 0 45N/mm^2 During the process the pressure of gas inside the balloon is proportional to cube of the diameter of the ballon Fin
- A spherical balloon of 1m diameter contains a gas 150 Kpa the gas inside the balloon heated util pressure reaches 450kpa find work done
- a gas undergoes the following reversible non flow process during its initial pressure of 15 bar and volume 1m^3/Kg to finalpressure of 6 bar
- a sphere ballon of 1 m dia contain a gas at 150 kpa
- A 0 8 diameter sphere contains helium at 20 bar
- a 1 5 diameter spherical balloon containing air
- a balloon is 1 m in diameter & contains air at 25°C
- a balloon is 1 meter in diameter and contains air at 25°c and 1 bar pressure if is filled with air isothermally and reversibly until the pressure reaches 5 bar assume pressure is proportional to diameter of balloon
- a balloon is 1m in diameter
- a balloon with 20 cm radius thermodynamics
- a 1 5m diameter spherical balloon containing air at 215 kpa
- a gas ballon has a diameter of 6 meters
- A gas confined a cylinder by a piston is at a pressure of 3 bar
- a gas system compressed by a piston and cylinder undergoes a change of state such that the product of pressure and the volume remains constant if the process begins at a pressure of 300 KPa and volume of 0 015 m3 and proceeds until the pressure falls to h
- a mass of 1 5 kg of air compressed in quasi static process from 1 bar to 7 bar
- A mass of 1 5 kg of air is compressed in a quasi-static process from 0 1 MPa to 0 7 MPa for which pv = constant The initial density of air is 1 16 kg/m3 Find the work done by the piston to compress the air
- a mass of 1 5kg of air is compressed in a quasi-static process from 0 1mpa to 0 7mpa for which pv=constant find the workdone by the piston to compress the air when the initial density of air is 1 16kg/m3
- a mass of 1 5kg of air is compressed in quasi static process from 0 1mpa to 0 7 mpa for which pv constant the initial density of air is 1 16kg/m3 find the work done
- A mass of 2 kg of air is compressed from 1 bar to 3 bar according to the law pv1 3 = constant The initial density of air is 1 5 kg/m3 Find the work done by the piston to compress the air
- a non flow quastic process if p=-3v 16 find work done
- a non reversible process occours for which pressure and volume are correlated by p=v^2 8/v