Other Types of Work Transfer
1. Shaft Work:
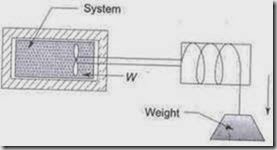
2. Stirring Work: Stirring work is nothing but shaft work is done on the system by using a stirrer which is driven by a shaft.
Figure3: Paddle-wheel work
As the weight is lowered, and the paddle wheel turns, there is work transfer into the system which gets stirred. Since the volume of the system remains constant, ò pdv = 0. If m is the mass of the weight lowered through a distance dz and T is the torque transmitted by the shaft in rotating through an angle dq, the differential work transfer to the fluid is given by ![]()
and the total work transfer is ![]() where W1 is the weight lowered
where W1 is the weight lowered
3. Electrical Work: When a current flows through a resistor, taken as system, there is work transfer into the system. This is because the current can drive a motor, the motor can drive a pulley and the pulley can raise a weight.
This is the rate at which work is transferred.
4. Work done in stretching a wire: Consider a wire as the system. If the length of the wire in which there is a tension Ŧ is changed from L to L + dL, the infinitesimal amount of work that is done is equal to, dw = – Ŧ dL
The -ve sign is used because a positive value of dL means an expansion of the wire, for which work must be done on the wire i.e., negative work.
5. Work done in changing the area of a surface film: A film on the surface of a liquid has a surface tension which is a property of the liquid and the surroundings. The surface tension acts to make the surface area of the liquid a minimum. It has the unit of force per unit length.
The work done on a homogeneous liquid film in changing its surface area by an infinitesimal amount dA is
6. Magnetization of a paramagnetic field: The work done per unit volume on a magnetic material through which the magnetic and magnetization fields are uniform is,
Note: It may be noted in the above expressions that the work is equal to the integral of the product of an intensive property and the change in its related extensive property. These expressions are valid only for infinitesimally slow quasi-static process.
Network Transfer: The network interaction between the system and the surroundings for any process will be the algebraic sum of all types of work interaction that has taken place between the system and the surroundings.
Therefore if W1-2 represents the net work transfer then,
+ve sign has to be used when the work transfer takes place from the system to the surroundings and –ve sign to be used when work transfer is from the surroundings to the system.
Heat: Heat is a mode of energy transfer that takes place between the system and the surroundings solely due to the temperature difference. Thus, heat is a transient phenomenon. It can be recognized only during a process. It is not a thermodynamic property of the system. Like work, heat is a path function i.e., the magnitude of heat transfer between the system and surroundings depends upon the type of process the system is undergoing.
Heat transfer always takes place from a region of higher temperature to a region of low temperature. The magnitude of the heat transfer into unit mass of the fluid in the system during a process from state (1) to state (2) will be written as ![]()
![]() represents the total heat transfer that takes place when the system undergoes a change of state from state 1 to state 2.
represents the total heat transfer that takes place when the system undergoes a change of state from state 1 to state 2.
Heat transfer is considered as positive if it takes place from the surroundings to the system and it is considered as negative if it takes place from the system to the surroundings.
During an adiabatic process, Q = 0
Units: Since heat is a form of energy transfer it will have the same units as that of energy. In SI units it is expressed in Joules (J) or Kilo Joules (kJ).
Comparison between work and heat:
Similarities:
-
Both are path functions and inexact differentials.
-
Both are boundary phenomenon i.e., both are recognized at the boundaries of the system as they cross them.
-
Both represent transient phenomenon; these energy interactions occur only when a system undergoes change of state i.e., both are associated with a process, not a state. Unlike properties, work or heat has no meaning at a state.
-
A system possesses energy, but not work or heat.
-
Concepts of heat and work are associated not with a ‘store’ but with a ‘process’.
Dissimilarities:
-
Heat is energy interaction due to temperature difference only; work is by reasons other than temperature difference.
-
In a stable system, there cannot be work transfer; however there is no restriction for the transfer of heat.
-
The sole effect external to the system could be reduced to rise of a weight but in the case of a heat transfer other effects are also observed.
-
Heat is a low grade energy whereas work is a high grade energy.
Problems:
1. Evaluate the work done in the following processes. The systems to be considered are underlined.
a) An agent slowly raises a body of mass 2 kg a distance of 3 mts in a gravitational field of standard acceleration.
Solution: By definition, considering agent as the system, it does positive work. The magnitude of work is measured by the product of the weight its lifts and the distance through which it is lifted.
W = + mg .l = 2 x 9.81 x 3 = + 58.86 J
Work done by the agent = + 58.86 J
Because, work is done by the agent, work is done on the body to the same amount.
Work done on the body = 58.86 J
or Work done by the body = – 58.86 J
b) A mass of 1 tonne is suspended from a pulley block. An agent slowly raises the mass against the standard gravitational acceleration by 2m.
Solution: By definition, considering agent as the system, it does positive work.
W = + mg .l = 1000 x9.81 x2 = 19620 J = 19.62 kJ
Work done by the agent = 19.62 kJ
Because, work is done by the agent, work is done on the mass to the same amount.
Work done on the mass = 19.62 kJ
or Work done by the mass = – 19.62 kJ
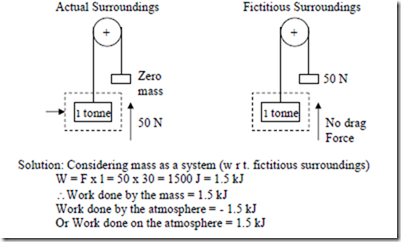
c) After raising the mass as in (b), the mass falls freely through the same vertical distance of 2 m. The drag force of the atmosphere on the body is 50 N.
d) A body of mass 15 kg falls freely in a vacuum through a vertical distance of 30m. The gravitational acceleration is 6 m/s2.
Solution: Considering body as a system, as it is falling freely there is no interaction with the system boundary and hence work done by the body is zero. (In other words, the work done by the body is zero as it can lift no weight. All that is happening as the body is falling freely is that its PE is decreasing and its KE is increasing accordingly).
e) A rat weighing 5.0 N climbs a stair 0.2 m in height.
Solution: Wrat = 0 (since there is no interaction between the system and its surroundings).
2. Indicate in the following cases, the heat exchange and work exchange are positive, negative or zero, and why
a) A copper block of 1 kg heated to 1000 C is dipped into water at 150 C. Consider copper as system.
Ans: δW = 0, δQ is negative
b) Heat is added to a gas in a rigid container such that pressure and temperature increases.
Consider gas as system,
Ans: δW = 0, δQ is positive.
c) Gas from a bottle is used to inflate a balloon which is originally flat. Consider gas as system.
Ans: δW is Positive, δQ = 0
d) An insulated wire is stretched. Consider wire as a system.
Ans: δW is negative, δQ = 0
e) A mouse climbs 20 steps of a stair case. Consider mouse as the system.
Ans: δQ = δW = 0
f) Gas in an insulated cylinder expands as the piston is slowly moved outwards.
Ans: δQ = 0, δW is positive
g) A closed rigid vessel containing steam at a temperature of 2000C is left standing in an atmosphere which is at 200C. Consider steam as the system.
Ans: For a closed rigid vessel, there is no change in volume and accordingly work done is zero. i.e., δW = 0. Since the steam is at a temperature higher than that of the surrounding atmosphere, the heat is rejected to the atmosphere. i.e., heat interaction is negative or δQ is negative.
h) The air in a tyre and connecting pump the pump plunger is pushed down, forcing air into the tyre. The tyre, pump walls and connecting tube can be thought of to be non- conducting. Consider air as a system.
Ans: δW is negative and δQ = 0
i) An electric current flows steadily through a resistor which is immersed in running water. Ans: Considering resistor as system, current flows through the resistance i.e., electrical work is done on the system δWe is negative
Due to the flow of current the resistor gets heated up resulting in heat transfer to the
surrounding cold water from the resistor.
δQ is negative (heat transfer from the system)
j) A container with rigid non-conducting walls holds a complete electrical circuit consisting of a heating element and charged storage battery. The temperature and pressure of the air in the container increases.
Ans: No interaction taking place across the boundary. The system boundary does not move as the walls are rigid. δW = 0
The walls are non-conducting though the temperature inside the system increase, no heat transfer to the surroundings can take place δQ = 0
k) 0.1 kg of gas contained in an insulated cylinder expands moving the piston slowly outwards
Ans: The cylinder is insulated No. heat transfer is possible δQ = 0
The gas expands the system boundary expands δWd is positive
Related posts:
Incoming search terms:
- types of work transfer
- Forms of work transfer
- work transfer
- forms of worktransfer
- Cylinder piston Resistor pump or fan work in Thermodynamics
- work transfer in thermodynamics
- work transfer and its types
- shaft work
- note on types of work transfer
- modes of work transfer
- gas in an insulated cylinder expands as the piston is slowly moved outward
- different types of work transfer
- work transfer types