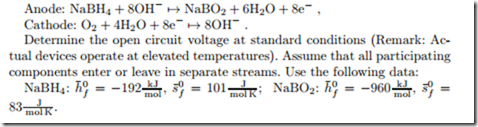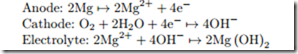Problems
Fuel Cell Potential
Compute the maximum voltage (reversible operation) for the fuel cell depicted below under the assumption that the oxygen supplied is five times the stoichiometric amount. Assume isothermal operation and assume that the water leaves as vapor.
Fuel Cell Potential and Power
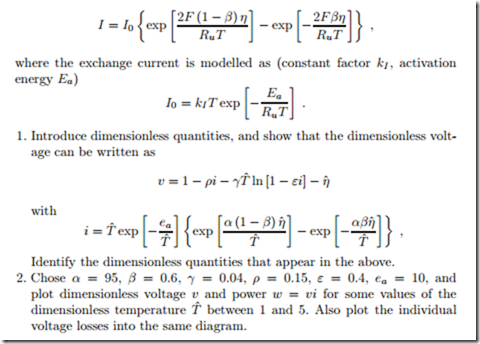
We can write the fuel cell voltage as V = V0 − VR − Vtr − η, where V0 is the open circuit potential, VR = RI are the ohmic losses due to the internal resistance R of the fuel cell, and Vtr = −BT ln (1 − I/Ilimit) is the potential drop due to mass transfer loss. Moreover the activation overpotential η is related to the current I through the Butler-Volmer equation
Electrolyzer
An electrolyzer can be considered as an “inverted fuel cell”: it consumes electric power and produces hydrogen and oxygen gases. For the following, ignore mass transfer contributions. Obviously, the current in the electrolyzer flows in the opposite direction: replace I by −I in the fuel cell equations of the previous problem. Show that now resistance and activation increase the fuel cell potential. Use the same constants as for the fuel cell, and plot the electrolyzer potential over the current, and also the power consumed as a function of current.
Direct Methanol Fuel Cell
In a direct methanol fuel cell, liquid methanol CH3OH reacts with oxygen to form water and carbon dioxide. The half-reactions at anode and cathode follow the equations
Determine the open circuit voltage at 330K. Assume methanol is an in- compressible liquid with constant specific heat c¯CH OH = 0.082 kJ .
Borohydride Fuel Cell
The reactions in a direct borohydride fuel follow the equations
Electrolyzer
Compute the voltage of a reversible electrolyzer that splits liquid water into hydrogen and oxygen. Assume that all incoming and outgoing streams are at reference pressure p0 = 1 atm and have a temperature of 360 K.
Direct Carbon Fuel Cell
The anode and cathode reactions in a direct carbon fuel cell follow the equations
Determine the open circuit voltage at 900 K, which is the typical operating temperature of actual DCFC’s. Assume carbon (graphite) is a incompressible substance with specific heat cp = 0.71 J .
Magnesium Fuel Cell
The reactions in a Magnesium fuel cell with salt water electrolyte follow the equations
Determine the open circuit voltage at standard conditions (Remark: Actual devices operate at elevated temperatures).
Molten Carbonate Fuel Cell
Molton carbonate fuel cells (MCFC) employ a mixture of salt and molten carbonate as electrolyte, and operate at temperatures around 900 K.
MCFC can work with carbon monoxide (CO) as fuel. Then the anode reaction occurs in two steps, first the water gas shift reaction, followed by reaction of the generated hydrogen with the carbonate ion, CO2−. The reactions occurring are:
1. Make a sketch of a fuel cell, where you indicate the relevant flows.
2. Determine the open circuit voltage at standard conditions and at 900 K.