Problems
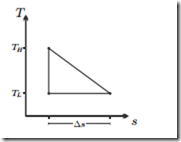
Power Cycle in the T-s-Diagram
Compute the efficiency of the cycle in the sketch, and compare with the efficiency of the Carnot cycle.
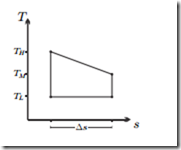
Refrigeration Cycle in the T-s-Diagram
A closed system runs on the process indicated in the sketch as a refrigeration cycle. Is the cycle running clockwise or counterclockwise? Use the sketch to determine the COP and compare with the Carnot cycle. Draw the p-v- diagram of the cycle for the case that the working fluid is an ideal gas. Assume TM = (TH + TL)/2.
Inventors
Two inventors have developed heat engines that operate between the temperatures of 900 K and 300 K. One inventor claims an efficiency of 50% for his engine, the other claims an efficiency of 66%. If you had some money to invest, which inventors start-up would you invest in? Explain!
Carnot Heat Engine
A heat engine operates on a fully reversible Carnot cycle between two reservoirs at 25 ◦C and 527 ◦C. The engine contains 4 g of air, and the largest volume is 2 litres. The pressure ratio for isothermal compression is equal to the pressure ratio for isentropic compression.
1. Draw the process diagrams, and make a table with temperature, pressure, specific volume, and specific internal energy at the corner points of the process.
2. Determine specific heat and work for the four processes in the cycle, the net work for the cycle, and the thermal efficiency.
3. When the engine runs at 350 rpm, determine the power produced.
Carnot Cycle
A Carnot power cycle using hydrogen as working fluid operates between the temperatures 320 K and 1000 K with maximum and minimum pressures given by 40 bar and 0.2 bar, respectively. The maximum volume of the hydrogen in the engine is 6.65 litres and the engine runs at 700 rpm. Draw the process in a T-s-diagram and in a p-v-diagram, then make a table with pressures, specific volumes, internal energies, and entropies at all corner points of the process. Compute the thermal efficiency, the mass of hydrogen in the engine, the maximum volume ratio, and the power produced. Based on the data obtained, discuss the feasibility of the process (apart from the fact that it would be impossible to build a fully reversible engine).
Carnot Heat Pump
A food drying unit requires a heating power of 200 kW which must be supplied at TH = 75 ◦C, while the exterior temperature is TL = 15 ◦C. For this purpose consider a closed system Carnot heat pump using xenon (ideal gas, monatomic,) as working fluid. The ratio between maximum and minimum volume of the unit is 40, and the largest pressure that can occur is 80 bar.
1. Draw the process in a T-s-diagram and in a p-v-diagram.
2. Make a table with pressures, temperatures and specific volumes, at all corner points.
3. Compute heat and work for all processes. Determine the net work and the COP.
4. When the engine operates at 500 rpm, determine the mass of xenon in the engine and the maximum volume (which could be distributed over several cylinders). Based on the data obtained, discuss the feasibility of the process.
Otto Cycle
The air entering a 3 litre Otto engine (ideal air-standard cycle, air as ideal gas with variable specific heats) with compression ratio r = 9.2 is at en- vironmental conditions (98 kPa, 300 K). After the heat supply, the pressure has doubled. Determine heat added, net work output, thermal efficiency, and power output when the engine runs at 2000 rpm. Draw diagrams.
Otto Engine
An air standard four-stroke Otto engine has a swept volume of 2.5 litres, and a clearance volume of 0.4 litres. Temperature and pressure at intake are given by T1 = 290 K, p1 = 0.7 bar. The temperature at the end of combustion is T3 = 1400 K. Consider the working fluid to be air, as ideal gas with R = 0.287 kJ , and with variable specific heats.
1. Draw the p-V- and T-s-diagrams for the process.
2. Determine the values of temperature, pressure, specific volume, and internal energy at the corners of the cycle. Make a table with these values.
3. Determine the net work per unit mass and the thermal efficiency.
4. Determine the mass of air in the cylinders and net power output of the engine when it runs at 4500 rpm.
Otto Engine
An air standard four-stroke Otto engine has a compression ratio of 9.4 and a clearance volume of 0.3 litre. Temperature and pressure at intake are T1 = 280 K, p1 = 1.1 bar, and the pressure after expansion is p4 = 2.984 bar.
Consider the working fluid to be air, as ideal gas with variable specific heats.
1. Draw the p-V- and T-s-diagrams for the process.
2. Determine the values of temperature, pressure, specific volume, and internal energy at the corners of the cycle. Make a table with these values.
3. Determine the net work per unit mass and the thermal efficiency.
4. Determine the mass of air in the cylinders and net power output of the engine when it runs at 1200 rpm.
Diesel Engine
An air standard four-stroke Diesel engine has a swept volume of 7 litres, and a clearance volume of 0.5 litre. The volume at the end of the fuel injection (isobaric heat supply) is 1 litre.
Temperature and pressure at intake are given by T1 = 300 K, p1 = 1 bar. Consider the working fluid to be air, as ideal gas with constant specific heats,
1. Draw a p-V-diagram of the process and then determine:
2. The values of all temperatures, pressures and specific volumes at the corners of the cycle. Make a table with these values.
3. The net work per unit mass and the thermal efficiency.
4. The mass of air in the cylinder and net power output of the engine when it runs at 2500 rpm.
Diesel Cycle
A 16 cylinder 170-litre Diesel engine operating on the ideal air-standard Diesel cycle has a compression ratio of 17, and a cut-off ratio of 2.2. Determine the amount of power delivered when the engine runs at 900 rpm based on the air- standard cycle, under cold-air assumption (that is: constant specific heats). Consider the following two cases for outside temperature and pressure: (a) T0 = 280 K, p = 1 bar. (b) T = 305 K, p = 0.9 bar. Draw diagrams.
Diesel and Otto cycle
Draw a schematic, and the process curves in a p-V-diagram and a T-s-diagram for a Diesel and an Otto cycle. Mark swept volume, clearance volume etc. These are four-stroke engines: what are the four strokes? Indicate them in the diagram. Discuss the difference between Diesel and Otto cycles. Why can the Diesel have a higher compression?
Dual Cycle
The processes in a 4-stroke Diesel cycle with compression ratio of 14 are modeled as dual cycle with the following data: The engine draws air at 1 bar, 27 ◦C, the maximum temperature reached in the cycle is 2200 K, and the total amount of heat added is q23 = 1520.4 kJ . The working fluid can be considered as air (ideal gas, variable specific heats).
1. Draw the process in a p-V-diagram, and a T-s-diagram. Include intake and exhaust, and indicate the 4 strokes in the p-V-diagram.
2. Determine temperatures, pressures, and specific volumes in the points 1,2,3’,3,4.
3. Determine the thermal efficiency of the cycle.
Atkinson Engine
A four-stroke-engine operating on the Atkinson cycle draws air at 0.9 bar, 17 ◦C. The working cycle consists of the following reversible processes:
1-2: Adiabatic compression of air with compression ratio 8.6
2-3: Isochoric heating to T3 = 1500 K
3-4: Adiabatic expansion
4-1: Isobaric cooling
Consider the working fluid to be air, as ideal gas with R = 0.287 kJ , and variable specific heats.
1. Draw p-v- and T-s-diagram for the cycle.
2. Make a list with temperature, pressure, specific volume at the four corner points of the cycle.
3. Determine the expansion ratio of the engine.
4. Determine net work and thermal efficiency of the cycle.
5. The engine runs at 2000 rpm and the engine delivers 28.75 kW. Determine the gas volume at bottom dead center.
Atkinson Engine
A four-stroke-engine operating on the ideal Atkinson cycle draws air at 0.9 bar, 17 ◦C. The working cycle consists of the following reversible pro- cesses:
1-2: Adiabatic compression of air with compression ratio 10.08.
2-3: Isochoric heating.
3-4: Adiabatic expansion with expansion ratio 16.
4-1: Isobaric cooling.
Consider the working fluid to be air, as ideal gas with R = 0.287 kJ , and variable specific heats.
1. Draw p-V- and T-s-diagram for the cycle.
2. Make a list with specific volume, temperature, pressure, at the four corner
points of the cycle.
3. Determine net work and thermal efficiency of the cycle.
4. The engine runs at 2000 rpm and the engine delivers 18 kW. Determine the mass in the cylinders, and the swept volume.
Overheated Atkinson Engine
Show that the thermal efficiency of the overheated Atkinson cycle under the cold-air approximation is given by Eq. (8.39).
Overheated Atkinson Engine
An overheated 4-stroke Atkinson cycle has a compression ratio of 10, and an expansion ratio of 14.09; its swept volume is 1.2 litres. The cycle draws air at p = 0.9 bar, T0 = 0 ◦C, and the total heat rejected into the environment is 397.38 kJ . Assume air-standard conditions, with air as ideal gas with variable specific heat, and reversible processes.
1. Draw the process into p-V- and T-s-diagrams.
2. Determine pressure, temperature, specific volume, specific internal energy and specific enthalpy at all relevant process points.
3. Determine the net work, the heat addition, and the thermal efficiency of the cycle.
4. The engine runs at 1750 rpm, determine the power output.