Philosophy of Thermodynamics
Thermodynamics
Thermodynamics is the set of laws that govern the properties of matter at the macroscopic scale. As such, there is a direct link between thermodynamics and our own daily experience. Indeed, we human beings are equipped with sensory apparatus designed to directly measure two of the most important thermodynamic quantities—namely, temperature and pressure. So this gives us a deeply ingrained notion of what these quantities are, or at least how they should behave.
This, however, is a mixed blessing, because from a scientific perspective, Recall the discussion in there are other causes at play—less tangible and more abstract, and with a molecular origin—that govern how temperature and pressure behave, and what they really are. Thus, whereas our sensory intuition can in some cases be “right,” in other cases, it can be wrong or misleading, and thereby lead us astray. Ultimately, mastery of thermodynamics—and science in general— requires looking at concepts beyond those of direct sensory experience.macroscopic or classical thermodynamics. On the other hand, we now know that the Laws of Thermodynamics ultimately arise from a molecular origin (see Section 2.3), and it is here that we must turn for a deeper, more rigorous understanding.
Molecularly speaking, macroscopic systems are extremely large and complex. Much of the utility of thermodynamics stems from the fact that it reduces all of this enormous molecular complexity down to just a few macroscopic parameters, known as thermodynamic variables (see Sec- tion 3.1). The net result is a greatly simplified description. In effect, the thermodynamic philosophy is to worry only about the “big picture,” and not about what each individual molecule is doing.
Much of the richness of—and interest in—thermodynamics lies in reconciling the macroscopic and molecular viewpoints. For example, it is interesting to contemplate just where exactly it is that inherently macroscopic thermodynamic quantities come from, from a molecular standpoint. Temperature and pressure, for instance, are perfectly well-defined quantities for bulk matter, yet it is impossible to assign values for these quantities to individual molecules, nor even to a small number of molecules. For this rea- son, such quantities are sometimes called “emergent properties,” meaning that precise values only “emerge” when the number of molecules becomes very large.
Such musings—though not what this book is really about—are difficult to avoid entirely in a conceptual treatment of thermodynamics. Rather, we seek primarily to provide a conceptual framework inasmuch as this serves to promote practical mastery of the subject across all disciplines. (Consult Chapter 17 and the website for a discussion of discipline-specific material.)
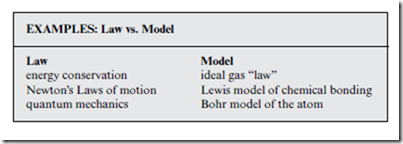
Scientific Models & Laws
In science and engineering, it is important to distinguish models from laws. Models are simplified, empirical descriptions of reality, built up over time in specific disciplines, based on observation of many specific examples. A scientific model is like a model car or airplane—lacking much of the detail and functionality of the original, and thus, not an exact description. In contrast, laws are always exact and true, because they derive from the fun- but “always” is of course a damental principles of physics. Both models and laws are important, but they play different roles, and should be interpreted differently.
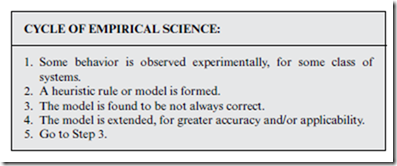
Empirical science typically works something like the cycle presented in the box below. Step 3 happens for the same reason that courts do not always convict properly, and pundits do not always predict the future correctly— because models are based on empirical evidence, rather than fundamental principles. In any case, this can lead to a never-ending cycle, wherein the model is perpetually improved, but never to the point of completely evading all doubt and/or controversy.
Interjecting the laws of physics into the above cycle can help “cut to the chase” and resolve ambiguity. In contrast to models, new laws do not come around very often, and they are effectively irrefutable. On the other hand, for our purposes, anyway…
laws also have their drawbacks. For example, they can be extraordinarily difficult to apply to real-world systems. Imagine, for instance, trying to simultaneously solve Newton’s equations of motion for Avogadro’s number’s worth of particles!
Are the principles of thermodynamics “laws” or “models”? Thermodynamics turns out to be a very, very special case, in that it provides the best of both worlds:
• It is an exact law of science, powerful and universal, yet…
• It is extremely simple, like a model.
The reason it can get away with this can be summarized in two words: statistical mechanics.
Statistical Mechanics
Thermodynamics is a law because it stems from a deeper theory called statistical mechanics that itself derives from the fundamental laws of physics. Specifically, statistical mechanics relates the macroscopic laws of thermodynamics to the physical laws governing the motion of individual molecules. The latter laws correspond to the “mechanics” part of statistical mechanics. This is taken to be either “classical” mechanics, for which Newton’s Laws are used to describe the molecular motion, or “quantum mechanics,” a more accurate and advanced treatment.
The “statistical” part of statistical mechanics is equally important. This is the notion that for very large systems, individual molecules have very little impact on the macroscopic behavior—the “big picture” philosophy, alluded to in Section 2.1. In order to relate the molecular and macroscopic viewpoints, statistical mechanics relies on statistical averages, which for our purposes belong to one of two types. The first type is an average over all molecules or particles in the system—or over the states available to those molecules (see Section 3.3). The second type is an average over time, possibly of a single molecule (or even a single molecular coordinate; see Sections 5.2 and 5.4).
Although thermodynamics “rests on top” of statistical mechanics, the two can be almost completely separated—rather like “mind” and “brain.” The idea of a total separation—essentially the purely macroscopic viewpoint—is undoubtedly appealing. However, a true conceptual under- standing requires at least some delving down to the molecular level. We therefore adopt a “middle-of-the-road” approach, wherein a greatly simplified statistical mechanics is employed to define a few core thermodynamic quantities such as temperature and entropy. As always, the main goal is conceptual clarity, rather than mathematical rigor. In any event, in our treatment, the two “lobes” of thermodynamics and statistical mechanics remain fused into a single whole.
Albert Einstein once remarked:
[Statistical thermodynamics] is the only physical theory of universal content which I am convinced will never be overthrown…
Why might he have said this? Perhaps because, despite being a mathematically very advanced topic, conceptually, statistical mechanics is really about nothing more than just counting—and the Law of Counting will never change.