Thermistors
A thermistor is a semi-conducting material – such as mixtures of oxides of copper, manganese, cobalt, etc. – in the form of a fused bead connected to two leads. As its temperature is increased its resistance rap- idly decreases. Typical resistance/temperature curves for a thermistor and common metals are shown in Figure 25.6. The resistance of a typical thermistor can vary from 400 W at 0°C to 100 W at 140°C.
Advantages
The main advantages of a thermistor are its high sen- sitivity and small size. It provides an inexpensive method of measuring and detecting small changes in temperature.
Pyrometers
A pyrometer is a device for measuring very high temperatures and uses the principle that all substances emit radiant energy when hot, the rate of emission depending on their temperature. The measurement of thermal radiation is therefore a convenient method of determining the temperature of hot sources and is particularly useful in industrial processes. There are two main types of pyrometer, namely the total radiation pyrometer and the optical pyrometer.
Pyrometers are very convenient instruments since they can be used at a safe and comfortable distance from the hot source. Thus applications of pyrometers are found in measuring the temperature of molten metals, the interiors of furnaces or the interiors of volcanoes. Total radiation pyrometers can also be used in conjunction with devices which record and control temperature continuously.
Total radiation pyrometer
A typical arrangement of a total radiation pyrometer is shown in Figure 25.7. Radiant energy from a hot source, such as a furnace, is focused on to the hot junction of a thermocouple after reflection from a concave mirror. The temperature rise recorded by the thermocouple depends on the amount of radiant energy received, which in turn depends on the temperature of the hot source. The galvanometer G shown connected to the thermocouple records the current which results from the e.m.f. developed and may be calibrated to give a direct reading of the temperature of the hot source. The thermocouple is protected from direct radiation by a shield as shown and the hot source may be viewed through the sighting telescope. For greater sensitivity, a thermopile may be used, a thermopile being a number of thermocouples connected in series. Total radiation pyrometers are used to measure temperature in the range 700°C to 2000°C.
Optical pyrometers
When the temperature of an object is raised sufficient- ly, two visual effects occur; the object appears brighter
and there is a change in colour of the light emitted. These effects are used in the optical pyrometer where a comparison or matching is made between the bright- ness of the glowing hot source and the light from a filament of known temperature.
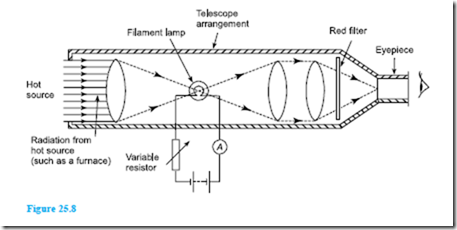
The most frequently used optical pyrometer is the disappearing filament pyrometer and a typical arrangement is shown in Figure 25.8. A filament lamp is built into a telescope arrangement which receives radiation from a hot source, an image of which is seen through an eyepiece. A red filter is incorporated as a protection to the eye.
The current flowing through the lamp is controlled by a variable resistor. As the current is increased, the temperature of the filament increases and its colour changes. When viewed through the eyepiece the filament of the lamp appears superimposed on the image of the radiant energy from the hot source. The current is varied until the filament glows as brightly as the background. It will then merge into the background and seem to disappear. The current required to achieve this is a measure of the temperature of the hot source and the ammeter can be calibrated to read the temperature directly. Optical pyrometers may be used to measure temperatures up to, and even in excess of, 3000°C.
Advantages of pyrometers
(i) There is no practical limit to the temperature that a pyrometer can measure.
(ii) A pyrometer need not be brought directly into the hot zone and so is free from the effects of heat and chemical attack that can often cause other measuring devices to deteriorate in use.
(iii) Very fast rates of change of temperature can be followed by a pyrometer.
(iv) The temperature of moving bodies can be mea- sured.
(v) The lens system makes the pyrometer virtually independent of its distance from the source.
Disadvantages of pyrometers
(i) A pyrometer is often more expensive than other temperature measuring devices.
(ii) A direct view of the heat process is necessary.
(iii) Manual adjustment is necessary.
(iv) A reasonable amount of skill and care is required in calibrating and using a pyrometer. For each new measuring situation the pyrometer must be re-calibrated.
(v) The temperature of the surroundings may affect the reading of the pyrometer and such errors are difficult to eliminate.
Temperature indicating paints and crayons
Temperature indicating paints contain substances which change their colour when heated to certain temperatures. This change is usually due to chemical decomposition, such as loss of water, in which the change in colour of the paint after having reached the particular temperature will be a permanent one. How- ever, in some types the original colour returns after cooling. Temperature indicating paints are used where the temperature of inaccessible parts of apparatus and machines is required. They are particularly useful in heat-treatment processes where the temperature of the component needs to be known before a quenching operation. There are several such paints available and
most have only a small temperature range so that different paints have to be used for different temperatures. The usual range of temperatures covered by these paints is from about 30°C to 700°C.
Temperature sensitive crayons consist of fusible solids compressed into the form of a stick. The melting point of such crayons is used to determine when a given temperature has been reached. The crayons are simple to use but indicate a single temperature only,
i.e. its melting point temperature. There are over 100 different crayons available, each covering a particular range of temperature. Crayons are available for temperatures within the range of 50°C to 1400°C. Such crayons are used in metallurgical applications such as preheating before welding, hardening, annealing or tempering, or in monitoring the temperature of critical parts of machines or for checking mould temperatures in the rubber and plastics industries.
Bimetallic thermometers
Bimetallic thermometers depend on the expansion of metal strips which operate an indicating pointer. Two thin metal strips of differing thermal expansion are welded or riveted together and the curvature of the bimetallic strip changes with temperature change. For greater sensitivity the strips may be coiled into a flat spiral or helix, one end being fixed and the other being made to rotate a pointer over a scale. Bimetallic ther- mometers are useful for alarm and over-temperature applications where extreme accuracy is not essential. If the whole is placed in a sheath, protection from cor- rosive environments is achieved but with a reduction in response characteristics. The normal upper limit of temperature measurement by this thermometer is about 200°C, although with special metals the range can be extended to about 400°C.
Mercury-in-steel thermometer
The mercury-in-steel thermometer is an extension of the principle of the mercury-in-glass thermometer. Mercury in a steel bulb expands via a small bore capillary tube into a pressure indicating device, say a Bourdon gauge, the position of the pointer indicating the amount of expansion and thus the temperature. The advantages of this instrument are that it is robust and, by increasing the length of the capillary tube, the gauge can be placed some distance from the bulb and can thus
inaccessible to the liquid-in-glass thermometer. Such thermometers may be used to measure temperatures up to 600°C.
Gas thermometers
The gas thermometer consists of a flexible U-tube of mercury connected by a capillary tube to a vessel containing gas. The change in the volume of a fixed mass of gas at constant pressure, or the change in pressure of a fixed mass of gas at constant volume, may be used to measure temperature. This thermometer is cumbersome and rarely used to measure temperature directly, but it is often used as a standard with which to calibrate other types of thermometer. With pure hydrogen the range of the instrument extends from –240°C to 1500°C and measurements can be made with extreme accuracy.
Choice of measuring devices
Problem 3. State which device would be most suitable to measure the following:
(a) metal in a furnace, in the range 50°C to 1600°C
(b) the air in an office in the range 0°C to 40°C
(c) boiler flue gas in the range 15°C to 300°C
(d) a metal surface, where a visual indication is required when it reaches 425°C
(e) materials in a high-temperature furnace in the range 2000°C to 2800°C
(f) to calibrate a thermocouple in the range –100°C to 500°C
(g) brick in a kiln up to 900°C
(h) an inexpensive method for food processing applications in the range –25°C to –75°C.
(a) Radiation pyrometer
(b) Mercury-in-glass thermometer
(c) Copper-constantan thermocouple
(d) Temperature sensitive crayon
(e) Optical pyrometer
(f) Platinum resistance thermometer or gas thermometer
(g) Chromel-alumel thermocouple
(h) Alcohol-in-glass thermometer.
Practise Exercise 139 Short-answer questions on the measurement of temperature
For each of the temperature measuring devices listed in 1 to 10, state very briefly its principle of operation and the range of temperatures that it is capable of measuring.
1. Mercury-in-glass thermomete.
2. Alcohol-in-glass thermometer.
3. Thermocouple.
4. Platinum resistance thermometer.
5. Total radiation pyrometer.
6. Optical pyrometer.
7. Temperature sensitive crayons.
8. Bimetallic thermometer.
9. Mercury-in-steel thermometer.
10. Gas thermometer.
Practise Exercise 140 Multiple-choice questions on the measurement of temperature
(Answers on page 298)
1. The most suitable device for measuring very small temperature changes is a
(a) thermopile
(b) thermocouple
(c) thermistor
2. When two wires of different metals are twisted together and heat applied to the junction, an e.m.f. is produced. This effect is used in a thermocouple to measure:
(a) e.m.f.
(b) temperature
(c) expansion
(d) heat
3. A cold junction of a thermocouple is at room temperature of 15°C. A voltmeter connected to the thermocouple circuit
indicates 10 mV. If the voltmeter is calibrated as 20°C/mV, the temperature of the hot source is:
(a) 185°C
(b) 200°C
(c) 35°C
(d) 215°C
4. The e.m.f. generated by a copper- constantan thermometer is 15 mV. If the cold junction is at a temperature of 20°C, the temperature of the hot junction when the sensitivity of the thermocouple is
0.03 mV/°C is:
(a) 480°C
(b) 520°C
(c) 20.45°C
(d) 500°C
In Questions 5 to 12, select the most appropriate temperature measuring device from this list.
(a) copper-constantan thermocouple
(b) thermistor
(c) mercury-in-glass thermometer
(d) total radiation pyrometer
(e) platinum resistance thermometer
(f) gas thermometer
(g) temperature sensitive crayon
(h) alcohol-in-glass thermometer
(i) bimetallic thermometer
(j) mercury-in-steel thermometer
(k) optical pyrometer.
5. Over-temperature alarm at about 180°C.
6. Food processing plant in the range –250°C to +250°C.
7. Automatic recording system for a heat treating process in the range 90°C to 250°C.
8. Surface of molten metals in the range 1000°C to 1800°C.
9. To calibrate accurately a mercury-in-glass thermometer.
10. Furnace up to 3000°C.
11. Inexpensive method of measuring very small changes in temperature.
12. Metal surface where a visual indication is required when the temperature reaches 520°C.
Revision Test 8 Hydrostatics, fluid flow, gas laws and temperature measurement
This Revision Test covers the material contained in Chapters 22 to 25. The marks for each question are shown in brackets at the end of each question.
When required take the density of water to be 1000 kg/m3 and gravitational acceleration as 9.81 m/s2.
1. A circular piston exerts a pressure of 150 kPa on a fluid when the force applied to the piston is 0.5 kN. Calculate the diameter of the piston, correct to the nearest millimetre. (6)
2. A tank contains water to a depth of 500 mm.
Determine the water pressure
(a) at a depth of 300 mm, and
(b) at the base of the tank. (6)
3. When the atmospheric pressure is 101 kPa, calculate the absolute pressure, to the nearest kilopascal, at a point on a submarine which is 50 m below the sea water surface. Assume that the density of sea water is 1030 kg/m3. (5)
4. A body weighs 2.85 N in air and 2.35 N when completely immersed in water. Determine
(a) the volume of the body
(b) the density of the body, and
(c) the relative density of the body. (9)
5. A submarine dives to a depth of 700 m. What is the gauge pressure on its surface if the density of seawater is 1020 kg/m3 and g = 9.81 m/s2. (5)
6. State the most appropriate fluid flow measuring device for the following applications:
(a) A high accuracy, permanent installation, in an oil pipeline.
(b) For high velocity chemical flow, which does not suffer wear.
(c) To detect leakage in water mains.
(d) To measure petrol in petrol pumps.
(e) To measure the speed of a viscous liquid. (5)
7. A storage tank contains water to a depth of 7 m above an outlet pipe, as shown in Figure 23.12 on page 268. The system is in equilibrium until a valve in the outlet pipe is opened. Determine the initial mass rate of flow at the exit of the out- let pipe, assuming that losses at the pipe entry = 0.3 v2, and losses at the valve = 0.2 v2. The pipe diameter is 0.05 m and the water density, r, is 1000 kg/m3. (15)
8. Determine the wind pressure acting on a slender building due to a gale of 150 km/h that acts perpendicularly to the building. Take the density of air as 1.23 kg/m3. (5)
9. Some gas occupies a volume of 2.0 m3 in a cylinder at a pressure of 200 kPa. A piston, sliding in the cylinder, compresses the gas isothermally until the volume is 0.80 m3. If the area of the piston is 240 cm2, calculate the force on the piston when the gas is compressed. (5)
10. Gas at a temperature of 180°C has its volume reduced by a quarter in an isobaric process. Determine the final temperature of the gas. (5)
11. Some air at a pressure of 3 bar and at a temperature of 60°C occupies a volume of 0.08 m3. Calculate the mass of the air, correct to the nearest gram, assuming the characteristic gas constant for air is 287 J/(kg K). (5)
12. A compressed air cylinder has a volume of 1.0 m3 and contains air at a temperature of 24°C and a pressure of 1.2 MPa. Air is released from the cylinder until the pressure falls to 400 kPa and the temperature is 18°C. Calculate
(a) the mass of air released from the container, and
(b) the volume it would occupy at S.T.P. Assume the characteristic gas constant for air to be 287 J/(kg K). (10)
13. A platinum resistance thermometer has a resistance of 24 W at 0°C. When measuring the temperature of an annealing process a resistance value of 68 W is recorded. To what temperature does this correspond ? Take the temperature coefficient of resistance of platinum as 0.0038/°C (5)
14. State which device would be most suitable to measure the following:
(a) materials in a high-temperature furnace in the range 1800°C to 3000°C.
(b) the air in a factory in the range 0°C to 35°C.
(c) an inexpensive method for food processing applications in the range –20°C to –80°C.
(d) boiler flue gas in the range 15°C to 250°C.
Related posts:
Incoming search terms:
- what instrument can be used to measure temperature between 1000 celsus to 1500 celsus
- mcq on temperature measurement
- Optical pyrometer
- rubbing thermocouple 1500 degree C
- how to measure 3000 degrees Celsius
- Pyrometer question and anserd
- types of pyrometer
- Radiation pyrometer
- Electric Heating Furnace Heat Treatment Furnace Bright Annealing manufactures
- which instrument is usede measure temperaturee above 350℃
- which of the following is most suitable for measuring very high temperatures
- which instruments is used to measure temperature above 350 degree c
- Which instrument you reccomment to use to measure temperature above 350 c
- device used to measure very high temperature
- device used to measure very high temperature is
- which instrument is used to measure temperature above 350 degree Celsius
- optical pyrometer 2000 degree
- which instrument is used to measure high temperature
- what type of temperature measure device used in boiler furnace
- Pyrometer question anserd
- Whic metal used measure high temperature in thermometer
- Radiation pyrometer objective questions
- how to measure tempreture over 2000 degree celcius
- Instrument used to measure high temperature
- thermometer used to measure temperature above 2000
- which instrument you recommend to use to measure temprature above 350 celcius
- list of instruments used to measure temperature
- photos of optical pyrometer
- to measure temperature above 1800 c the most suitable instrumemt
- which instrument is used to measure the temperature above 300°c
- mcq question which instrument you recommend to use to measure temperature above 350 degrees Celsius
- device for measuring temperature in a boiler
- 3000 centigrad mersuring meter
- a thermometer suitable for a measuring a temperature of the order 5000°c
- device used to measure high temperature
- •Which instrument is used for measuring high temperatures
- for measurement high tmepreture of furence the indtrume t is use
- for measurement of high Temperatures of funace the instrument used is
- for measurement of high temrature of furnace
- device is used to very high temperature is
- a Pyrometer can be used for measurement of mcq
- because3t4
- device that used to measure very high temeracture
- answer this question an instrument used to measure high temperature
- an instrument used for measuring the temperature of a very hot furnace
- Among mercury thermometer thermoelectric pyrometers platinum resistance thermometer Which is used to measure high temperature??
- device used to measure high temerature
- device used to measure a very high temperature gas
- device use to measure the temperature of boiler
- device use to measure high temperature