Freezing of a Liquid Solution
Ice crystals cannot easily accept salt molecules into their lattice, and thus when a salt solution is cooled, first water will freeze out, while the salt will remain in the liquid as long as possible. Sea-ice, that is ice that freezes out of oceans in cold climates, contains no salt. As sea-ice forms, the salt content of the water just below the ice increases. This salty water has increased density, and will sink towards the bottom of the ocean, thus driving ocean currents. Completely frozen salt-water solution is a mixture of pure ice and regions of salt-water crystals, e.g., NaCl-2H2O crystals.
The dissolved salt ions move in the liquid, they have a larger entropy when they can move in a larger volume, that is when more liquid water is present.
So in order to gain entropy, the dissolved salt prevents water from freezing, which is observed as a drop in the temperature at which water will freeze.
To estimate this drop, we consider solid and liquid as incompressible ideal mixtures, so that we can approximate the chemical potentials of the solvent in liquid solution (/) and ice (//) as
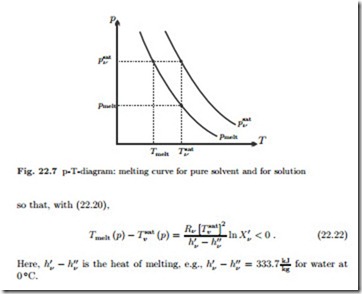
For water, the volume of ice is larger than the volume of liquid, and hence the melting pressure of the solution, pmelt (T ), is lower than that of pure water (psat (T )).
To compute the change in melting temperature, we use Fig. (22.7) and the Clausius-Clapeyron equation to estimate