Desalination in a Continuous Process
In the previous section we have seen that salt can draw water through a semipermeable membrane. In the inverse process, fresh water is obtained from saltwater, by pressing the saltwater against a semipermeable membrane, which only allows freshwater to pass, e.g., think of increasing the pressure on the saltwater column in the Pfeffer tube. We ask for the minimum work required for desalination, that is for the work required in a reversible process.
Figure 21.3 shows the general set-up for a continuous desalination plant, without specifying how desalination is taking place inside the plant, which is drawn as a grey box. A mole flow n˙ sw of salt water with salt mole fraction Xsw enters the plant at environmental pressure and temperature (p0, T0).
Work W˙ is supplied to the plant, which also exchanges heat Q˙
with the environment. Two streams leave the plant at (p0, T0), a stream of freshwater n˙ fw, and the brine stream n˙ b which contains all salt and has a salt mole fraction Xb > Xsw .
Since all flows are at (p0, T0), ΔG˙ 0 is the difference in Gibbs free energy at environmental conditions flowing through per unit time.
According to (21.4), for reversible operation, S˙gen = 0, the work required for desalination is given by −ΔG˙ 0. To simplify the problem we assume ideal mixtures, where μ¯α (T0, p0, Xβ ) = g¯α (T0, p0) + R¯T0 ln Xα, and find the minimum work as
where Δs¯mix is the entropy of mixing for mixing brine and freshwater per mole of mixture, i.e., for one mole of seawater. It should be noted that the assumption of ideal mixture will not hold for large salt content, thus this is a hypothetical value.
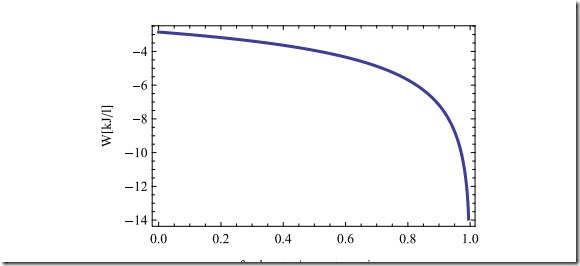
Figure 21.4 shows the work required as function of the freshwater ratio y.
For smaller values of y the increase in work requirement is not too large, and one might consider to go to values of y = 0.5, or so. Note that larger values of y reduce the amount of seawater drawn for a given amount of freshwater produced, and thus the overall size of the device or plant. As y approaches the maximum value, we observe fast increase of the work requirement.
The work requirement will be larger in actual processes, where additional work input is needed to overcome irreversible losses. For a membrane-based desalination system there are losses to friction in the pipes, the pump, in filters, and as water is pressed through the membrane, and losses due to non- uniform salt concentration: salt might accumulate in front of the membrane and this concentration polarization increases the local osmotic pressure and thus the work requirement. Note that less work is needed when reverse osmosis takes place at lower temperatures, where the osmotic pressure is lower. Typical values for separation work in commercial membrane desalination systems are about 10 kJ per litre of freshwater.