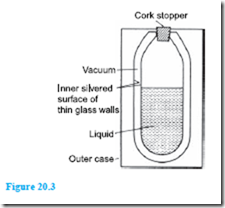
Vacuum flask
A cross-section of a typical vacuum flask is shown in Figure 20.3 and is seen to be a double-walled bottle with a vacuum space between them, the whole sup- ported in a protective outer case.
Very little heat can be transferred by conduction be- cause of the vacuum space and the cork stopper (cork is a bad conductor of heat). Also, because of the vacuum space, no convection is possible. Radiation is minimised by silvering the two glass surfaces (radiation is reflected off shining surfaces).
Thus a vacuum flask is an example of prevention of all three types of heat transfer and is therefore able to keep hot liquids hot and cold liquids cold.
Use of insulation in conserving fuel
Fuel used for heating a building is becoming increasingly expensive. By the careful use of insulation, heat can be retained in a building for longer periods and the cost of heating thus minimised.
(i) Since convection causes hot air to rise it is important to insulate the roof space, which is probably the greatest source of heat loss in the home. This can be achieved by laying fibre-glass be- tween the wooden joists in the roof space.
(ii) Glass is a poor conductor of heat. However, large losses can occur through thin panes of glass and such losses can be reduced by using double- glazing. Two sheets of glass, separated by air, are used. Air is a very good insulator but the air space must not be too large otherwise convection currents can occur which would carry heat across the space.
(iii) Hot water tanks should be lagged to prevent conduction and convection of heat to the surrounding air.
(iv) Brick, concrete, plaster and wood are all poor conductors of heat. A house is made from two walls with an air gap between them. Air is a poor conductor and trapped air minimises losses through the wall. Heat losses through the walls can be prevented almost completely by using cavity wall insulation, i.e. plastic-foam.
Besides changing temperature, the effects of supplying heat to a material can involve changes in dimensions, as well as in colour, state and electrical resistance.
Most substances expand when heated and contract when cooled, and there are many practical applications and design implications of thermal movement as explained in Chapter 21 following.
Now try the following Practise Exercises
Practise Exercise 110 Short-answer questions on heat energy
1. Differentiate between temperature and heat.
2. Name two scales on which temperature is measured.
3. Name any four temperature measuring devices.
4. Define specific heat capacity and name its unit.
5. Differentiate between sensible and latent heat.
6.The quantity of heat, Q, required to raise a mass m kg from temperature t1° C to t2 °C, the specific heat capacity being c, is given by Q = ……
7. What is meant by the specific latent heat of fusion?
8. Define the specific latent heat of vaporisation.
9. Explain briefly the principle of operation of a simple refrigerator.
10. State three methods of heat transfer.
11. Define conduction and state two practical examples of heat transfer by this method.
12. Define convection and give three examples of heat transfer by this method.
13. What is meant by radiation? Give three uses.
14. How can insulation conserve fuel in a typical house?
Practise Exercise 111 Multiple-choice questions on heat energy
1. Heat energy is measured in:
(a) kelvin
(b) watts
(c) kilograms
(d) joules
2. A change of temperature of 20°C is equivalent to a change in thermodynamic temperature of:
(a) 293 K
(b) 20 K
(c) 80 K
(d) 120 K
3. A temperature of 20°C is equivalent to:
(a) 293 K
(b) 20 K
(c) 80 K
(d) 120 K
4. The unit of specific heat capacity is:
(a) joules per kilogram
(b) joules
(c) joules per kilogram kelvin
(d) cubic metres
5. The quantity of heat required to raise the temperature of 500 g of iron by 2°C, given that the specific heat capacity is 500 J/(kg °C), is:
(a) 500 kJ
(b) 0.5 kJ
(c) 2 J
(d) 250 kJ
6. The heat energy required to change 1 kg of a substance from a liquid to a gaseous state at the same temperature is called:
(a) specific heat capacity
(b) specific latent heat of vaporisation
(c) sensible heat
(d) specific latent heat of fusion
7. The temperature of pure melting ice is:
(a) 373 K
(b) 273 K
(c) 100 K
(d) 0 K
8. 1.95 kJ of heat is required to raise the temperature of 500 g of lead from 15°C to its final temperature. Taking the specific heat capacity of lead to be 130 J/(kg °C), the final temperature is:
(a) 45°C
(b) 37.5°C
(c) 30°C
(d) 22.5°C
9. Which of the following temperature is absolute zero ?
(a) 0°C
(b) – 173°C
(c) – 273°C
(d) – 373°C
10. When two wires of different metals are twisted together and heat applied to the junction, an e.m.f. is produced. This effect is used in a thermocouple to measure:
(a) e.m.f.
(b) temperature
(c) expansion
(d) heat
11. Which of the following statements is false?
(a) – 30°C is equivalent to 243 K
(b) Convection only occurs in liquids and gases
(c) Conduction and convection cannot occur in a vacuum
(d) Radiation is absorbed by a silver surface
12. The transfer of heat through a substance by the actual movement of the particles of the substance is called:
(a) conduction
(b) radiation
(c) convection
(d) specific heat capacity
13. Which of the following statements is true?
(a) Heat is the degree of hotness or cold- ness of a body.
(b) Heat energy that flows to or from a sub- stance while the temperature remains constant is called sensible heat.
(c) The unit of specific latent heat of fusion is J/(kg K).
(d) A cooker-grill is a practical application of radiation.