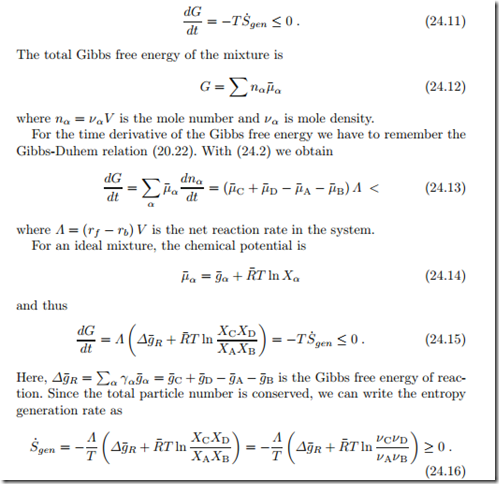Entropy Generation
We show that with the reaction laws (24.5,24.9) the entropy generation is always positive as the reaction approaches towards equilibrium, and that it is zero in equilibrium. For simplicity of the argument, we consider only the reaction (24.1) as before.
When the reaction takes place in a closed system the first and second laws read
We restrict the discussion to the case where temperature and pressure are kept constant throughout the reaction. Then W˙ dV and the two laws can be combined by elimination of the heat Q˙ to
In thermodynamic equilibrium the term in the bracket, and thus the entropy generation rate, vanishes: this gives the law of mass action (24.7).
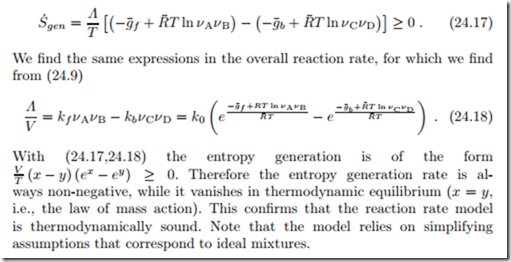
With Δg¯R = g¯f − g¯b we can split this term into contributions referring to the forward and backward reactions,