PHOTOVOLTAIC DEVICES
Photovoltaic devices, often called solar cells, are solid-state devices that are capable of absorbing sunlight and converting it into an electrical current. The earliest observation of the photovoltaic effect was by the French scientist Antoine-Ce´sar Becquerel but the practical solar cell was developed by scientists at Bell Laboratories in the United States in the 1940s and 1950s. These cells
were around 6% efficient and during the 1960s they were used in the U.S. space program. As costs came down they began to be used in the 1980s for remote terrestrial applications and during the 1990s, with prices continuing to fall, grid-connected solar cells began to appear. Their use expanded dramatically from the end of the 1990s, and by the beginning of the second decade of the 21st century they had become the third most important source of renewable electricity after hydropower and wind power. Their simplicity, combined with continued lowering costs, means that they could overtake wind power in the next decade or two.
Solar Photovoltaic Technology
The operation of a solar cell depends on a fundamental property of a semiconductor called its bandwidth. The bandwidth is a function of the semiconductor’s atomic structure that results in an energy gap between the top layer of full elec- tron energy levels and the first set of empty energy levels. The energy gap is too large for electrons to jump from the lower to the upper as a result of thermal activation. However, electrons in the lower level can become promoted from the lower to the upper level, across the bandwidth, by absorbing photons of elec- tromagnetic radiation. For this to be possible, the photon must contain at least as much energy as the size of the energy gap between the two sets of energy levels in the semiconductor.
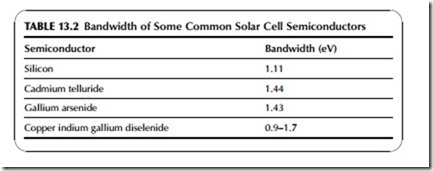
The range of electromagnetic radiation that the cell can absorb is determined by the size of its bandwidth. Semiconductors that are useful for solar cells have bandwidths that make them capable of absorbing photons within the visible region of the solar spectrum, but any photon with an energy lower than the band- width (e.g., infrared radiation) will not be absorbed. However, all those with energy greater than the bandwidth can be absorbed. Table 13.2 shows the band- widths of some semiconductors that are commonly used for solar cells.
Each photon of light energy that is absorbed by the semiconductor is captured by an electron within the material. In absorbing the energy, the electron acquires an electrical potential (it has more energy than most of those around it). This potential can be made available as electrical energy, as an electric current.
The current is produced at a specific fixed voltage called the cell voltage. The cell voltage is, again, a property of the semi-conducting material. For silicon it is around 0.6 V.
The energy contained in light increases as the frequency increases from infrared through red to blue and ultraviolet light. However, a solar cell must throw away some of these frequencies since it can only absorb light above its cell threshold. Light that is of an energy below this threshold simply passes through the material.
It might seem sensible, therefore, to use a semiconductor with a low thresh- old or bandwidth. However, this will lead to a cell with a low output voltage since this is also directly related to the threshold for absorption. There is another drawback to using a semiconductor with a small bandwidth. When a photon of light with energy much greater than the threshold energy is absorbed, it loses all the energy above the threshold value. The surplus energy is essentially thrown away (it emerges as heat) and cannot be used for electricity production.
These two factors mean that the lower the bandwidth and absorption thresh- old, the more energy is thrown away as heat, but the higher the bandwidth, the more energy simply passes through the material without being absorbed. The optimum is, therefore, a compromise between the two competing effects.
The optimum bandwidth for a solar cell semiconductor is 1.43 eV. The bandwidth of silicon, at 1.11 eV, is below the optimum but it has proved the most effective solar cell material to date and has the largest market share. Sil- icon is used in three different forms: crystalline, semi-crystalline, and amor- phous. Crystalline silicon is the most efficient but also the most expensive to produce, while amorphous silicon is both the least efficient and the cheapest. Alternatives to silicon include cadmium telluride, which is cheaper to produce and is always in amorphous form. Its bandwidth is very close to the optimum. Other materials such as gallium arsenide are also more efficient that silicon, particularly in crystalline form, but are much more expensive.
Types of Solar Cell
Solar cells are manufactured using technologies similar to those used to manufacture microchips and transistors. Most of these are made using slices of per- fect silicon crystals that are then etched and doped to create the complex structures that are required for computers and other electronic devices. Solar cells, though normally simpler in structure than a microchip, can be manufac- tured in a similar way.
Silicon solar cells made from single crystal silicon are the most efficient available with reliable commercial cell efficiencies up to 20% and laboratory efficiencies measured at 24%. Even though this is the most expensive form of silicon it remains the most popular by a wide margin due to its high efficiency and durability. Polycrystalline silicon is cheaper to manufacture but the penalty is lower efficiency with the best measured around 18%. Cheapest of all to produce is amorphous silicon, which can be made using vapor deposition tech- niques rather than by expensive crystal growing. However, its best efficiency is only 8%. Amorphous silicon also suffers from degradation when first exposed to light, a feature not seen with crystalline material. This can reduce its initial efficiency by up to 20%.
All silicon solar cells require extremely pure silicon. The manufacture of pure silicon is both expensive and energy intensive. The traditional method of production required 90 kWh of electricity for each kilogram of silicon. Newer methods have been able to reduce this to 15 kWh per kilogram. This still means that depending on its efficiency, a silicon solar cell can take up to two years to generate the energy needed to make it. This compares with around five months for a solar thermal power plant. Manufacturers of crystalline silicon are concentrating on ways of reducing the cost of crystalline material by cutting it more efficiently or by finding new ways of growing it. This is helping push costs down, and crystalline silicon remains compet- itive in spite of efforts by thin film manufacturers using much cheaper materials.
Another crystalline material used for solar cells is gallium arsenide. This has an almost perfect bandwidth for a solar cell and in the laboratory efficiencies of 28% have been recorded. However, practical cells only reach 20% and the mate- rial is both expensive and composed of hazardous materials. It is rarely used, except for special applications.
The main alternative to crystalline silicon for solar cells is some form of thin film. From a manufacturing point of view these are attractive because they can be produced using cheap techniques such as vapor deposition or even printing. Amorphous silicon is one alternative but it is not as cheap to produce as cadmium telluride and the latter has a much higher efficiency, with the best recorded at 17% though commercial cells rarely achieve more than 11%. This material also has an almost optimum bandwidth for a solar cell (1.44 eV) and its potential efficiency could approach 30%. Cadmium telluride is also attractive because it is possible to produce solar cells on a variety of substrates including building components and flexible plastic sheets.
The maximum efficiency possible with a single-layer solar cell of any semi- conductor is 33.7%. It is possible to build more efficient solar cells by layering cells one on top of the other. Such multilayer or multijunction cells place the semiconductor with the largest bandwidth at the top. This will absorb high- energy radiation but that of lower energy will pass through it to reach the layers below where further semiconductor layers of lower bandwidth are placed. In principle, it is possible to create a cell with up to 50% energy efficiency with multilayer cells, but they are much more expensive to manufacture. The best recorded efficiency achieved to date is 43.7%.
Cell Structures
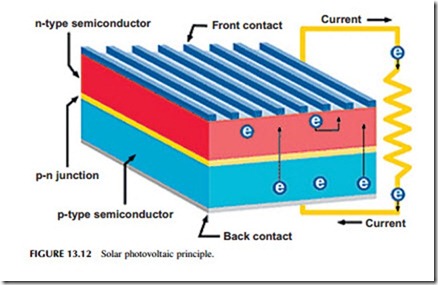
The conventional structure for a solar cell is planar (Figure 13.12). The cell is made of a thin layer of semiconductor, the top surface of which is doped with an
impurity to create a p-n junction within the body of the material. It is this junction, with a built-in voltage gradient, that captures electrons once they are generated by light absorption and sweeps them away to form an electric current. To collect the current, electrical contacts must be formed onto the semiconductor. The rear of the cell can be covered with a planar collector, but on the front sur- face, where light is absorbed, the collector area must be minimized or it will interfere with light absorption. The front collector is usually made from narrow fingers of metal that allow the maximum amount of sunlight to strike the semi- conductor surface. The semiconductor will often be placed on a substrate to give it additional strength and the top layer will be covered with a protective coating.
More advanced structures are possible. The front contacts can be buried, edgeways, into the surface of the cell to minimize the shadow effect of the con- tact that reduces efficiency. More modern cells have also been developed that move both contacts to the back of the cell by allowing the front doping to be carried to the back of the semiconductor. This structure is more complex but creates a more efficient cell.
Efficiency depends on light absorption. This can be improved by making the front surface of the cell nonreflecting and by making the back reflective so that any light reaching the back layer is reflected back into the semiconductor. Such developments mean that the number of stages in the fabrication of a solar cell can increase to perhaps 15 from a more usual 6–8. However, the lowering of production costs and the increased efficiency are making such developments worthwhile.
Such advanced techniques are generally only used with crystalline silicon. Amorphous or thin-film devices rely on cheaper fabrication techniques for their economics. Cadmium telluride, the most popular thin-film material, is usually formed on a substrate such as glass. However, it can also be deposited onto flexible plastics. These thin films can be produced in much larger areas than crystalline silicon, which helps make them more cost effective. Copper- indium-gallium-diselenide is another thin-film solar cell material. This has the advantage that by varying its composition, the bandwidth can be varied, which offers the potential to tailor cells to particular applications or to manufacture complex multilayer cells with specific characteristics.
Concentrating Solar Cells
Concentrating solar cells adopt a different strategy to planar cells. Whereas the latter capture sunlight over a large area of semiconductor, concentrating cells use cheap optical systems to concentrate the sunlight first, in a manner similar to most solar thermal technologies, before converting it into electricity. In principle, an optical system can be manufactured more cheaply than a large area planar solar cell. The device can then use a very expensive, but highly efficient,multijunction solar cell at the focus to convert the light into electricity. As noted before, this type of solar converter has achieved the highest efficiency of any solar cell at 43.7%.
The disadvantage of the concentrating cell is that it requires direct sunlight and cannot operate effectively with diffuse sunlight. While these converters have a limited market share today, there are signs that they may be attractive for utility-scale applications in high insolation regions where their high efficiency is likely to be an advantage.
Third-generation Solar Cells
In the market for solar cells, crystalline silicon cells are often referred to as first- generation solar cells and thin-film devices as second-generation cells. Third- generation cells are new organic and dye-sensitized solar cells.
Organic semiconductors operate in exactly the same way as conventional ones, but with the disadvantage that the organic material does not conduct electricity as readily as a traditional semiconducting material. The key to develop- ing organic materials is to find an efficient way of extracting the electrical power once it has been generated. (If the excited electrons are not swept away quickly, they will simply fall back to the lower energy level in the semiconduc- tor and lose their energy.) While this can be a drawback, the attraction of organic materials is the simplicity of their production. It is expected to be possible to print the semiconductor onto substrates, making it extremely flexible in the way it can be used. However, efficiencies of organic semiconductors are currently very low with the best laboratory value of 6% and the best production efficiency of 4%.
Dye-sensitized solar cells use special dyes to absorb light and then transfer the electron produced during the absorption to a substrate semiconducting material. These devices have been compared to photosynthesis, but the complex series of exchanges required to make them work also makes them appear similar to electrochemical devices such as batteries. As with organic semiconductors these are attractive because of the simplicity of manufacture as they too can be printed. Theoretical maximum efficiency is 31% and practical efficiencies of 13% have been achieved. Both types can maintain their outputs under low light conditions but long-term stability is still questionable.
Modules, Inverters, and Panels
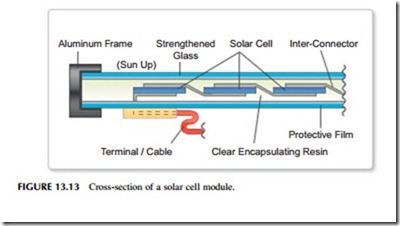
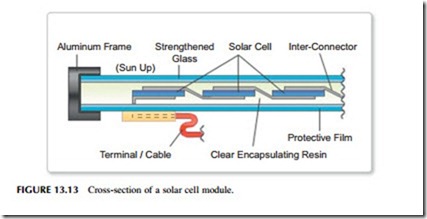
Most solar photovoltaic energy conversion is based on planar solar cells but the actual solar cells account for only a part of the complete system required to cre- ate a solar energy conversion installation. Individual silicon solar cells only pro- duce one of two watts of electricity each at, for a silicon cell, 0.6 V. Therefore, cells must be connected in series and in parallel to create modules capable of both higher power and of generating at a higher voltage (Figure 13.13). These modules must then be encapsulated to protect them from the environment. An encapsulated module of this type is called a solar panel and it may have an out- put of several watts to several hundred watts. Current encapsulation techniques are capable of providing modules with lifetimes of 25–30 years. Advanced encapsulation techniques may be able to extend this to perhaps 50 years of more in the near future.
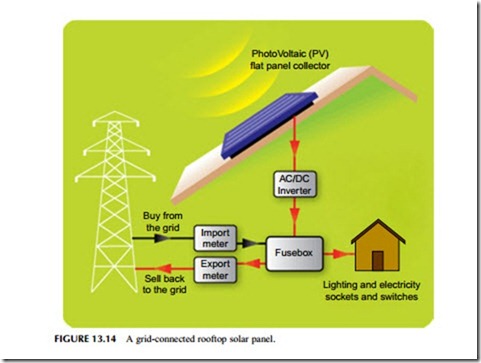
The output of the solar panel is direct current. This can be used without conditioning in some remote applications, but for grid connection the DC must be converted into alternating current at the grid voltage. This is carried out using a solid-state inverter. Modern inverters, particularly for larger solar panel installations, are sophisticated devices that can provide two-way communication with transmission and distribution system operators. Other parts of the complete
installation include the supports, typically of steel. For a modern solar photo- voltaic installation the solar cell probably accounts for 50–60% of the total cost, but this is falling as fabrication costs fall.
System Types
There are broadly four types of solar photovoltaic installation, each serving a different market sector. The most important of these today is the residential sec- tor that includes installations up to 20 kW on domestic rooftops (Figure 13.14). This is the sector that has been the focus of incentive schemes in countries such as Japan, Germany, the United Kingdom, Italy, and the United States, and it accounts for by far the largest installed capacity of solar cells, worldwide.
Allied to this is the commercial sector. This covers larger building installations at schools, hospitals, office buildings, shopping centers, and other similar organizational buildings. Installations for these types of buildings may be up to 1 MW in generating capacity.
The utility sector is the third major market sector. This involves large arrays feeding power directly into the grid. Plants of this type have capacities between
1 MW and 200 MW and larger installations are likely in the future. This is developing into one of the main markets for concentrating solar cells too.
The final market sector is for off-grid installations. This can range from small domestic installations to much larger utility-sized installations providing power for remote communities. In 2010 the residential sector accounted for 63% of the total market for solar cells and the utility sector for 19%. Commercial systems took 11% and off-grid systems the remaining 7%. The residential sector is expected to continue to dominate the market but the utility sector will grow strongly too over the next decade.
Solar Photovoltaic Generation and Energy Storage
As with solar thermal generation, there is a significant attraction in being able to combine solar cells and some form of energy storage. This can both extend the period during which the solar system can supply power and, where grid con- nected, provide a more reliable and therefore more valuable electricity source.
The most popular way of adding storage to a solar cell system is by means of batteries. Since the output of a solar cell is direct current and a battery is a DC device, the solar cell can be used to charge a battery system without any con- ditioning. In the past lead-acid batteries have been popular as part of off-gird solar-generating systems. Today there is a move toward using battery storage with grid-connected systems too so that the owners can take more power from their installation; several manufacturers are developing lithium-ion batteries to serve this market. Using a battery system with a grid-connected solar system is particularly attractive if the price obtained for power exported to the grid is much lower than the cost of buying power from the grid.
Battery storage is probably the easiest to implement with a solar generator but other approaches are possible. Small-scale heat storage, though relatively inefficient, can be used to harness excess solar power. For larger-scale production, systems such as hydrogen generation are feasible and even methane production.
As with all storage, much will depend on the relationship between the owner of the solar generator and the grid. It will always be more efficient, overall, to export surplus power to the grid where it can be used in a number of different ways than it will be to store it locally. Grid-level storage will generally be more efficient than local storage too although installation costs are high and this has tended to dissuade utilities from building grid storage in the past.