GASIFICATION AND PYROLYSIS
In recent years a number of companies have developed new WTE technologies based on both gasification and pyrolysis. These technologies are derived from the power and the petrochemicals industries. Pyrolysis is a partial combustion process carried out at moderate to high temperatures in the absence of oxygen and it can produce a mixture of gaseous, liquid, and solid residues. The traditional method of producing charcoal is a form of pyrolysis, as is the production of gas from coal which leaves a residue of coke. Gasification, meanwhile, involves heating solid material at high temperatures in a limited amount of air or oxygen to produce a char waste and a combustible gas. In both cases the gas will normally be burned to generate heat and steam.
Pyrolysis can produce a range of products from waste, depending on the temperature and the time the waste spends in the pyrolysis reactor. At lower temperatures with short residence times, more oils and tars are produced. Lon- ger residence times lead to more solid residue (char). When the temperature is low, these solid and liquid products can contain complex and sometimes toxic organic molecules and must generally then be combusted at a controlled high temperature to generate power in a conventional boiler system.
Many WTE pyrolysis plants operate at relatively high temperatures so that they do not produce any liquid or tar residues, only combustible gas and a solid residue. Waste is normally sorted first, removing and recycling as much metal, glass, and plastics as possible. The remainder is then shredded and reduced to small particles of perhaps 2 mm in size before exposing it to a high temperature of around 800 ºC to convert it very quickly into a combustible gas and solid ash. The gas, which has a calorific value up to 22 MJ/kg, is cleaned and can then be used in a gas engine to generate power. Alternatively, the gas can be used in a conventional boiler.
One of the main advantages touted for high-temperature pyrolysis over incineration is that production of toxic organic compounds, such as dioxins and furans, is minimized. One company’s pyrolysis WTE plant is designed to treat around 90 tonnes a day of waste with a calorific value of 8.4 MJ/kg and a moisture content of 40%, generating 2 MW of power in the process.6
Another, lower-temperature pyrolysis system, developed in the 1990s in Japan,7 employs an initial pyrolysis process followed by combustion to generate heat. Waste delivered to the plant is first shredded and then fed into a rotating pyrolysis drum where it is heated to around 450 ºC. The heat, provided by hot air generated at a later stage in the process, pyrolyses the waste, converting it into a combustible gas and a solid residue.
The solid residue contains any metal that entered with the waste. This can be removed at this stage for recycling. Both iron and aluminum can be segregated in this way. The remaining solid slag is crushed. The gas and the crushed residue are then fed into a high-temperature combustion chamber operating at 1300 oC where it is completely burned. Combustion is controlled to limit nitrogen oxide formation. Incombustible material adheres to the walls of the combustion cham- ber where it flows, in liquid form, to the bottom. From here it is led out of the bottom of the furnace and immediately quenched, creating an inert granular material suitable for road building.
Hot flue gases from the combustion chamber are used to generate steam to drive a turbine. Dust is then removed from the exhaust gases and returned to the combustion system. Following this, a flue-gas treatment system removes any remaining acid gases. Only this material, around 1% of the original volume of the MSW, needs to be disposed of in a landfill. This system also claims to keep residual levels of dioxins extremely low.
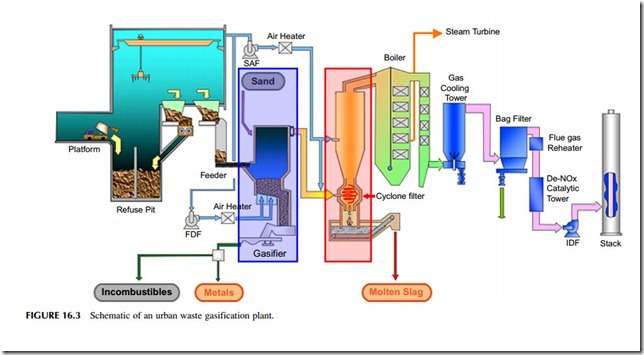
Waste gasification (Figure 16.3) is similar to pyrolysis but it generally takes place at a higher temperature, typically 1000 oC, to produce a combustible, low
calorific–value gas that can be burned either in a gas engine or in a conventional boiler system. As with pyrolysis, the products of gasification depend on the tem- perature used and lower temperatures can lead to more contaminants in the gas. The
synthetic gas produced during gasification is generally a mixture of carbon monoxide and hydrogen but the carbon monoxide can be converted into more hydrogen by using a shift reaction in which the gas is mixed with water vapor and passed over a catalyst at high temperatures. Provided it is clean enough, this gas can be used either for power generation or as a feedstock for industrial processes.
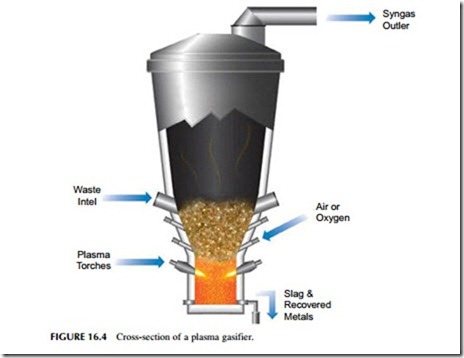
A new version of the gasification process that is being developed for MSW processing is plasma gasification (Figure 16.4). This involves burning the waste in a plasma arc at temperatures that can reach in excess of 10,000 oC, although the actual gasification will generally take place at slightly lower temperatures. Even so, the temperature is so high that all the components of the waste are bro- ken down to atomic constituents and the product is expected to be a relatively pure syngas.
A plasma gasification reactor consists of a gasification chamber with a plasma torch generating a high-temperature arc close to the bottom. Solid waste is introduced above the torch, and as it falls through the high-temperature region, it is gasified in the presence of a controlled amount of air introduced from the bottom of the chamber. Any metal and slag is removed from the bottom of the reactor while syngas exits from the top. The high temperatures reduce the levels of chemicals such as dioxins to a very low level, often much lower than the levels produced in a conventional incineration plant.
The syngas from a plasma gasifier can be burned in an engine or conventional combustion plant. It is also possible to feed the gas directly into a coal-fired power plant in the same way as was discussed for biomass gasification in Chapter 15.