EMISSION CONTROL FOR GAS TURBINE POWER PLANTS
The combustion of natural gas, the main fuel for gas turbines, has a relatively low environmental impact when compared to its main fossil fuel competitor, coal. After it has been cleaned it contains relatively little if any hydrogen sulfide and no heavy metals. Depending on the precise composition of the natural gas and on the gas turbine combustion system in which it is burned, its combustion will generate some carbon monoxide and also some particulate material, both resulting from incomplete combustion of components of the fuel. Aside from these, the main atmospheric pollutant of concern with gas turbine power generation is nitrogen oxide generated by oxidation of nitrogen in the combustor. Levels of carbon monoxide, particulates, and nitrogen oxide can peak during startup and under part-load operation.
Gas turbines also generate carbon dioxide from the combustion of the hydrocarbon fuel they burn. The quantity generated for each unit of electricity is much less than would be released from a coal-fired power station, but even so, large combined cycle power plants are major carbon dioxide emitters.
Nitrogen Oxide
Nitrogen oxide can be produced in gas turbines from two different sources. Small amounts of nitrogen can be found bound in the fuel itself as part of the complex mixture of hydrocarbon-based materials that can make up natural gas. This nitrogen, when it exists, will be converted into nitrogen oxide during combustion. However, the main source of nitrogen oxide is from nitrogen gas, either from the air in which the gas is burned or actually contained in small amounts with the fuel.
Most nitrogen oxide production is the result of the oxidation of nitrogen gas by oxygen in the air at the high temperatures that are reached in the combustor of the gas turbine. With power turbine inlet temperatures as high as 1600 oC or greater in large high-efficiency gas turbine combined cycle systems, this presents a significant problem. The solution has been to develop low nitrogen oxide burners that control the combustion in such a way as to limit nitrogen oxide production.
One successful technique that has been applied in a range of gas turbine combustors is water or steam injection. This serves to lower the combustion temperature and thereby reduce nitrogen oxide production. However, as temperatures have risen higher, these have been replaced by dry low nitrogen oxide burners that achieve the same end without water injection.
The simplest way to burn natural gas in air is to pump the gas through a fine nozzle where it is ignited as it enters the atmosphere. With unlimited amounts or air, and therefore of oxygen available, this diffusion or spray combustion results in a stable flame within which optimum combustion conditions will ultimately be reached, leading to complete combustion and limited formation of carbon monoxide and particulate material. However, this unrestrained combustion can also lead to high levels of nitrogen oxide.
The main alternative and the technique that is now used in most dry low nitrogen oxide burners is called premixed combustion. This involves premixing the natural gas with a carefully controlled quantity of air before it enters the combustion chamber, such that there is just sufficient oxygen to react with the combustible gas but none left to react with nitrogen. Controlling combustion under these conditions is much more difficult. If there is too little air then combustion is incomplete and will result in high concentrations of carbon monoxide and unburned material, leading to harmful emissions and lower efficiency because all the energy within the fuel is not utilized. On the other hand, if the amount of air becomes too high, then nitrogen oxide levels quickly rise.
Therefore, premixed combustion must be carefully controlled. When it is, modern low nitrogen oxide burners are capable of reducing the emissions of nitrogen oxide to between 15 ppm to 25 ppm for large industrial gas turbines and as low as 9 ppm for some smaller gas turbines. Nitrogen oxide emission standards are typically 25 ppm in many parts of the world and manufacturers aim to meet these standards without further control being necessary. However, standards are tightening and some areas are introducing emission limits of 15 ppm or 10 ppm for gas turbines. These are much harder for large combined cycle plants to meet without further nitrogen oxide reduction systems.
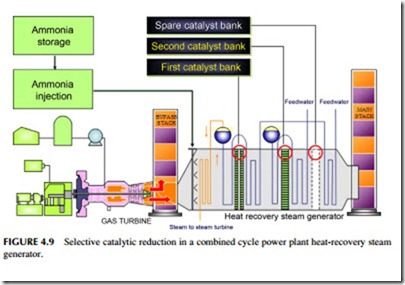
Where low nitrogen oxide burner technology cannot meet the required emission standard, manufacturers have to turn to an alternative post-combustion technology. The most common of these is selective catalytic reduction (SCR). SCR involves injecting a reducing gas, usually ammonia or, sometimes, urea, into the exhaust gases after they exit the power turbine and then passing the mixture over a metal catalyst that catalyzes the reaction between the ammonia, or urea, and nitrogen oxide to produce nitrogen and water vapor. In a combined cycle power plant the SCR unit is often placed within the heat-recovery steam generator as shown in Figure 4.9.
Catalysts for SCR are normally either a metal or metal oxide on a ceramic carrier. Various metals have been used, including vanadium, molybdenum, and tungsten, as well as platinum, though the latter is mostly found in the system used in car exhausts. The type of catalyst will depend on the temperature of the exhaust gases. Metal oxides are more temperature resistant than metals.
In principle, an SCR-based nitrogen oxide reduction system can remove 95–99% of the nitrogen oxide from the exhaust gases. However, the system becomes more difficult to control when reduction levels exceed 80% because the reaction does not proceed as smoothly, putting greater demands on the catalyst and often leading to higher levels of ammonia or urea passing through the system and being released into the atmosphere. This is referred to as ammonia slip.
Therefore, the optimum solution is a balance between low nitrogen oxide burners and SCR such that both operate within the best range of efficiency. With both, it is possible to reduce nitrogen oxide emission levels below 10 ppm and often much lower than this. Targets of 4–5 ppm appear to be achievable with modern systems.
Carbon Monoxide
Carbon monoxide can be generated in gas turbines as a result of incomplete combustion of the natural gas fuel. Emissions of carbon monoxide are controlled in the same way as those of nitrogen oxide with similar limits of
10–25 ppm in operation. Low nitrogen oxide burner technology can lead to levels of carbon monoxide higher than this, under which circumstances some system of emission control is needed. This is usually in the form of an oxidation catalyst that catalyzes the conversion of carbon monoxide into carbon dioxide. This may be a separate catalytic unit, but in combined cycle plants it is often incorporated into the heat-recovery steam generator too.
Carbon Dioxide
The combustion of natural gas generates significant quantities of carbon dioxide. Although the rate of production is much lower than in a coal-fired power plant, the gross level of production can be large. In the United States in 2009, for example, gas turbine–based power plants accounted for 23% of total power generation and 13% of carbon dioxide emissions.
For each kilowatt of energy contained in natural gas, its combustion will pro- duce 0.23 kg of carbon dioxide. For coal the equivalent figure is 0.37 kg/kWh. The actual emissions per unit of electricity produced will depend on the efficiency of electricity production, but a combined cycle plant operating at 55–60% efficiency will emit relatively less than a coal plant at 38–45% efficiency.
Combined cycle plants are considerably less carbon intensive than coal-fired plants, and this has been one of the driving forces behind a switch to more natural gas–fired power generation in the developed world. Even so, when car- bon capture from power plants becomes necessary, it is unlikely that combined cycle units will be exempt.
The main methods of reducing or eliminating carbon emissions from gas turbine–based power plants are exactly the same as those for coal-fired power plants that were discussed in Chapter 3: post-combustion capture, pre- combustion capture, and oxy-fuel combustion. The concentration of carbon dioxide in the flue gases from a typical combined cycle power plant will be 3–4%. This is much lower than in the flue gases of a coal-fired power plant and makes post-combustion capture relatively more difficult. On the other hand, the low concentration means that less needs to be removed.
Post-combustion capture using an ammonia or monoethanolamine absor- bent in a spray tower is likely to be the most effective form of post-combustion capture for combined cycle plants. It is also an efficient means of retrofitting capture technology. The problem of low carbon dioxide concentration in the exhaust gases can be mitigated by recycling exhaust gases back to the gas tur- bine inlet. This has the effect of increasing carbon dioxide concentration but also of reducing the available oxygen entering the combustion chamber. Exhaust gas recycling of up to 35% appears to be effective without adversely affecting the combustion conditions. With exhaust gas recycling, post- combustion capture should be able to remove 90% of the carbon dioxide generated. Overall energy conversion efficiency is reduced by perhaps 6–8%.
Pre-combustion capture is essentially analogous to the coal gasification technology discussed in Chapter 3, producing hydrogen fuel gas that is then burned in a gas turbine combined cycle power plant. In the case of natural gas the process is known as reforming and is widely carried out industrially to make hydrogen. As with post-combustion capture, the capture efficiency is likely to be around 90%, but the overall generation efficiency is likely to fall more, perhaps by 10%.
Oxy-fuel combustion involves burning the natural gas in pure oxygen instead of air. This requires an oxygen separation plant but leaves an exhaust gas that is rich in carbon dioxide and containing little nitrogen, making it much easier to isolate. Capture efficiency may be as high as 99%. As was the case in a coal-based oxy-fuel plant, the combustion temperature when natural gas is burned in oxygen is far higher than in air—too high for the materials currently available to withstand. To counter this, carbon dioxide–rich exhaust gases are fed back to the gas turbine inlet to dilute the oxygen, reducing the combustion temperature to a level similar to that for air combustion. Overall energy conver- sion efficiency is likely to fall by up to 8–9% compared to a gas turbine plant without capture.