FUEL CELL PRINCIPLE
The fuel cell principle belongs to a branch of chemistry called electrochemistry. This explores how electricity can be derived from a chemical reaction. In nature certain materials will react with one another spontaneously if the conditions are correct. For example, a strong acid like sulfuric acid will react vigorously with a variety of different materials if they are mixed with it. Other reactions will also proceed spontaneously but require a kick-start. Typical of these is the reaction of hydrogen with oxygen. The two gases can be mixed at ambient temperatures without any reaction, but once the temperature is raised beyond a certain point, with a spark for example, the reaction will proceed explosively.
Spontaneous reactions of this type are called exothermic reactions because they all release heat energy when they are allowed to proceed freely. All the chemical reactions that can be exploited to generate electrical energy are spontaneous reactions. However, when the reaction is exploited electrochemically, the progress of the reaction is managed in such a way that some of the energy that would normally be released as heat emerges instead as electrical energy.
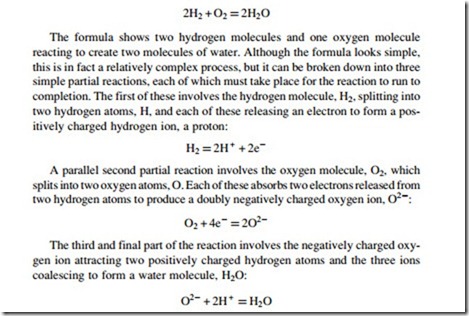
The reaction between hydrogen and oxygen is exothermic. This reaction can be expressed by a simple chemical formula:
When hydrogen burns in air, the various steps of the reaction occur in the same place at the same time. However, in a fuel cell the hydrogen and oxygen are not allowed to mix. Instead, the reacting gases are introduced separately with hydrogen supplied to one electrode of the cell and oxygen to the other. The two electrodes are separated by a material called the electrolyte.
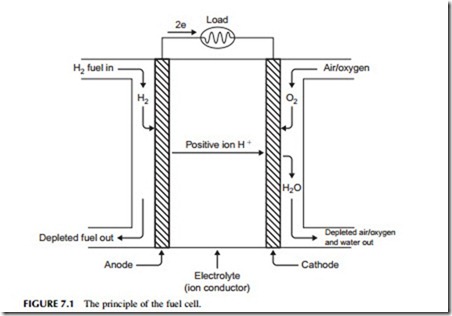
The electrolyte is the key element in any electrochemical cell (Figure 7.1) because it acts like a filter to both stop the cell reactants mixing directly with one another and to control how the charged ions created during the partial cell reactions are allowed to reach each other. The electrolyte in a fuel cell is impermeable to the gases, hydrogen and oxygen. It will not conduct electricity in the form of electrons either, nor (provided it is an acidic electrolyte) will it conduct the negatively charged oxygen ions. What it will do is conduct positively charged hydrogen ions.
At the hydrogen electrode (called the anode) hydrogen molecules first adhere to the electrode material and then separate into atoms, each subsequently releasing an electron to form a positively charged ion as shown in the preceding equations. In this ionic form the hydrogen can cross the electrolyte boundary and reach the oxygen at the second electrode. The electrons, however, are left behind at the electrode.
At the second electrode (called the cathode) oxygen atoms will also adhere to the electrode surface and dissociate, each leaving two oxygen atoms. How- ever, these require a supply of electrons if they are to form oxygen ions. Only in this form can they coalesce with the hydrogen ions traveling through the electrolyte from the anode to create water molecules. The electrons must come from the anode, but these electrons cannot pass through the electrolyte.
If an electrical connection is made between the two electrodes the electrons can now pass through the connecting wire to the oxygen atoms where they will create oxygen ions, allowing the reaction to run to completion. When a small electric light bulb is inserted into this circuit it will glow, proving that an electric current is indeed flowing between one electrode and the other.
All electrochemical cells operate in this way. While part of the reaction can take place within the confines of the cell, it can only be completed if electrons are allowed to travel from one electrode to the other through an external circuit.1 If no connection is made the exothermic nature of the reaction means that a charge builds up at each electrode, creating an electrical potential, the cell volt- age, which will drive electrons from one electrode to the other when a connection is made.
Since the electrolyte is such an important part of an electrochemical cell, fuel cells are generally identified by the type of electrolyte they employ.
Phosphoric acid fuel cells use phosphoric acid, which being acidic is a proton (positively charged hydrogen atom) conductor. Proton exchange membrane cells also rely on an acidic membrane to allow hydrogen ions to pass. The alkaline fuel cell is a hydroxide ion (OH-) conductor; the hydroxide ion is the complement in water of the hydrogen ion. Meanwhile, the high-temperature solid oxide fuel cell uses a solid that is a conductor of oxygen ions. Other fuel cell reactions appear more complex even though the end result is the same. So, for example, the molten carbonate fuel cell electrolyte conducts carbonate ions.