Storage batteries
Storage batteries accumulate electrical energy and release it on demand. The familiar lead-acid battery was invented more than 100 years ago by Gaston Plante. It suffers from poor energy density (watt-hours per pound) and poor power density (watts per pound). The average life is said to be in the neighborhood of 360 complete charge-discharge cycles. During charging, the lead-acid battery shows an effi- ciency of about 75%; that is, only three-quarters of the input can be retrieved.
Yet it remains the only practical alternative for automotive, marine, and most stationary engine applications. Sodium-sulfur, zinc-air, lithium-halide, and lithium- chlorine batteries all have superior performance, but are impractical by reason of cost and, in some cases, the need for complex support systems.
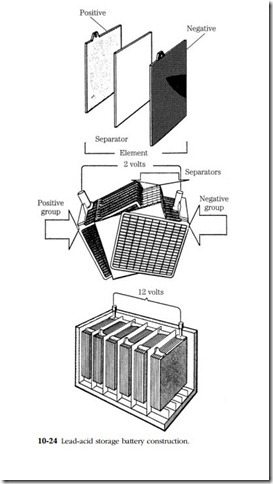
The lead-acid battery consists of a number of cells (hence the name battery) connected in series. Each fully charged cell is capable of producing 2.2V. The number of cells fixes the output; 12V batteries have six cells; 24V batteries have 12. The cells are enclosed in individual compartments in a rubberoid or (currently) high-impact plastic case. The compartments are sealed from each other and, with the exception of Delco and other “zero maintenance” types, open to the atmosphere. The lower walls of the individual compartments extend below the plates to form a sediment trap. Filler plugs are located on the cover and can be combined with wells or other visual indicators to monitor the electrolyte level.
The cells consist of a series of lead plates (Fig. 10-24) connected by internal straps. Until recently the straps were routed over the top of the case, making convenient test points for the technician. Unfortunately, these straps leaked current and were responsible for the high self-discharge rates of these batteries.
The plates are divided into positive and negative groups and separated by means of plastic or fiberglass sheeting. Some very large batteries, which are built almost entirely by hand, continue to use fir or Port Orford cedar separators. A few batteries intended for vehicular service feature a loosely woven fiberglass padding between the separators and positive plates. The padding gives support to the lead filling and reduces damage caused by vibration and shock.
Both sets of plates are made of lead. The positive plates consist of a lead grid- work that has been filled with lead oxide paste. The grid is stiffened with a trace of antimony. Negative plates are cast in sponge lead. The plates and separators are immersed in a solution of sulfuric acid and distilled water. The standard proportion is 32% acid by weight.
The level of the electrolyte drops in use because of evaporation and hydrogen loss. (Sealed batteries have vapor condensation traps molded into the roof of the cells.) It must be periodically replenished with distilled water. Nearly all storage bat- teries are shelved dry, and filled upon sale. Once the plates are wetted, electrical energy is stored in the form of chemical bonds.
When a cell is fully charged the negative plates consist of pure sponge lead (Pb in chemical notation), and the positive plates are lead dioxide (PbO2, sometimes called lead peroxide). The electrolyte consists of water (H2O) and sulfuric acid (H2SO4). The fully charged condition corresponds to drawing A in Fig. 10-25. During discharge both sponge lead and lead dioxide become lead sulfate (PbSO4). The percentage of water in the electrolyte increases because the SO4 radical splits off from the sulfuric acid to com- bine the plates. If it were possible to discharge a lead-acid battery completely, the elec- trolyte would be safe to drink. In practice, batteries cannot be completely discharged in the field. Even those that have sat in junkyards for years still have some charge.
During the charge cycle the reaction reverses. Lead sulfate is transformed back into lead and acid. However, some small quantity of lead sulfate remains in its crystalline form and resists breakdown. After many charge-discharge cycles, the residual sulfate permanently reduces the battery’s output capability. The battery then is said to be sulfated.
The rate of self-discharge is variable and depends on ambient air temperature the cleanliness of the outside surfaces of the case, and humidity. In general it aver- ages 1% a day. Batteries that have discharged below 70% of full charge might sulfate. Sulfation becomes a certainty below the 70% mark. In addition to the possibility of damage to the plates, a low charge brings the freezing point of the electrolyte to near 32°F.
Temperature changes have a dramatic effect on the power density. Batteries are warm-blooded creatures and perform best at room temperatures. At 10°F the battery has only half of its rated power.