Fuel cells
Like a battery, a fuel cell converts the energy of a chemical reaction directly into electrical energy. The process here involves the oxidation of an external fuel, which is normally a hydrogen-rich gas, and the reduction of an oxidant, which is usually atmospheric oxygen. Electrons are passed from the fuel electrode to the oxidant electrode through the externally connected load, and the electrical circuit is completed by ions that cross an electrolyte to produce water.
The fuel cell has a number of advantages over the conventional heat engine and shaft-driven generator for the production of electrical power. The generation efficiency is much higher in a fuel cell at scales even up to several megawatts, it has a higher power density, lower vibration characteristics, and reduced emission of pollutants. An individual fuel cell operates at a dc voltage of about 1 V. Cells must therefore be
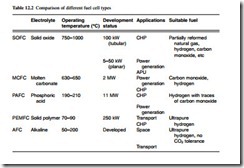
connected to stacks of series and parallel connection in order to deliver the voltage and power required for many applications. Another complication is that many fuel cells cannot operate effectively using a raw hydrocarbon fuel; most types require a reformer, which converts the hydrocarbon into a hydrogen-rich gas suitable for passing directly into the fuel cell. The main types of fuel cell are best classified according to temperature of operation and fuel requirement, as in Table 12.2.
The two main types of fuel cells being developed at present are the Solid Polymer Electrolyte Fuel Cell (PEFC) and the Solid Oxide Fuel Cell (SOFC). These two main classes are distinguished by the type of electrolyte they use. This in turn determines their operating temperature.
The Polymer Electrolyte Fuel Cell (PEFC)
An example of a PEFC is shown in Fig. 12.16. The solid polymer fuel cell is based upon a proton conducting polyfluorosulphonic acid membrane with finely dispersed platinum electrodes upon a porous carbon matrix. Machined carbon or steel plates are utilized as bipolar plates to form series stacks. The PEFC operates at temperatures below 100°C with humidified gases. It is a potentially clean method of generating electricity which is silent, robust and efficient. Applications for the PEFC cover the domestic, commercial and industrial range, but it will probably be best suited to small-scale Combined Heat and Power (CHP) applications, and for transport duties. The PEFC is often described with reference to power density, which ranges from 0.25–1.0 kW/l. Some PEFCs are commercially available for demonstration or early application at 1–5 kW for CHP and up to 25 kW for transport. Expected lifetime is still only a few thousand hours for the stack, but system lifetime is expected eventually to be in the range of 8–20 years.
The Solid Oxide Fuel Cell (SOFC)
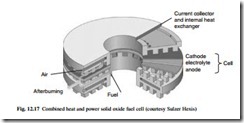
The SOFC is illustrated in Fig. 12.17. It typically comprises a nickel zirconia cermet anode, yttria stabilized zirconia electrolyte, lanthanum strontium manganite cathode and a chrome-based alloy interconnect. The SOFC operates at much higher tempera- tures than the PEFC, in the region of 850°C to 1000°C. It offers the possibility of high electrical efficiency together with high-grade exhaust heat; important applications for the SOFC are therefore in the combined heat and power field and in other generation applications where a significant heat load is present.
Advantages of the SOFC are very high electrical efficiency, the ability to utilize conventional fuels such as natural gas with limited processing and the facility to pro- duce steam or hot water from the high temperatures that are available. Typical power ratings are in the range 150–250 kW and life expectancy is 5–20 years, with adequate maintenance. The first demonstration systems in the range 1–2 kW (electrical output) have recently become commercially available, normally in combined heat and power with about 10 kW (thermal output).