Nature of light
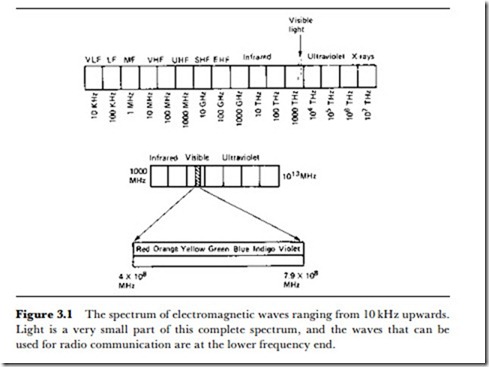
Light is an electromagnetic radiation of the same kind as radio waves, but with a very much shorter wavelength and hence a much higher frequency. The relation between wavelength, frequency and the velocity of electromagnetic waves is given by:
where A is wavelength in metres, V is frequency in Hz, and c is the velocity in mjs. The velocity of light in free space is 3 x 108 mjs.
The place of light in the range of possible electromagnetic waves is shown in Figure 3.1, along with an expanded view of the portion of the range which we class as infrared, visible and ultraviolet. This range of wave- lengths, i.e., the spectrum of electromagnetic waves, is the subject of this chapter, and the shorter wavelengths (higher frequencies) are considered separately from the longer wavelengths of electromagnetic (radio) waves for two important reasons. One is that this range of waves is not generated or detected by the conventional electronic methods that are used for waves in the millimetre to kilometre range.
The other point is that the very short wavelengths of this range cause effects that are not a problem when we work with radio waves. One such problem is coherence, mentioned earlier in conjunction with laser interferom- eters. Light from a small portion of a source, such as light passing through a pinhole, can be coherent over a short distance (a fraction of a millimetre), but only laser light is coherent over distances of a metre or more. Only coherent light exhibits the effects of interference which are so common with radio waves, though light is diffracted at edges and pinholes, causing spurious images.
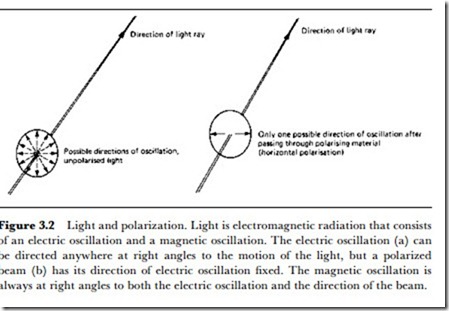
Like any other form of radiated wave, light can be poLarized, and this is a topic that is of importance in some applications. Normal unpolarized light consists of waves whose direction of oscillation can be in any plane at right angles to the direction of motion (Figure 3.2). When such light is passed
through a polarizing material, the light can become plane-polarized, meaning that the oscillation is in one plane of the material only.
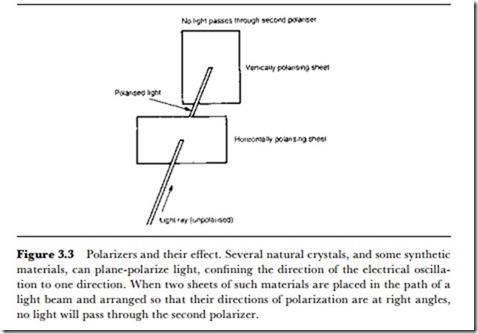
Materials that polarize light in this way are generally crystals or other materials that contain lines of atoms at a critical spacing. If polarized light is beamed on another sheet of polarizing material, the amount of light that passes through the material depends on the angle of the sheet, because two sheets of polarizing material with their planes of polarization at right angles will not pass any light (Figure 3.3). Like radio waves, light can also be polarized in other ways (circular, elliptical polarization), but plane polarization of light is the most common.
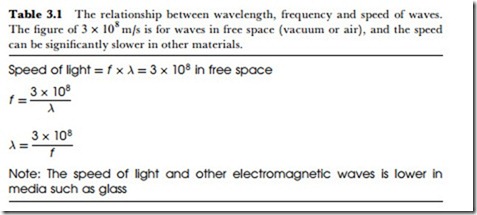
Like the other waves in the electromagnetic range, light (considering the broad range of infrared to ultraviolet) has a velocity in space that is fixed at about 3 x 108 mjs, so that the usual relationship between wavelength and frequency (Table 3.1) holds good. When light travels through transparent materials, however, the velocity is reduced, and the factor by which the velocity is reduced is called the refractive index, a number that is always greater than unity. The velocity of light in any transparent material is therefore equal to the free-space velocity of 3 x 108 divided by the refractive index value for the material. For visible light, refractive index values for materials range from just above unity to about 2 – the highest values are for gemstones, notably diamond.
Refractive index values for infrared can be greater than this, and materials that are opaque to visible light may be quite transparent to infrared – an example is rock-salt. This illustrates how critical the effect of
the wavelength of the radiation can be. In general, the interaction between an electromagnetic radiation and a material can be expected to be critical when the wavelength of the radiation is of a value similar to the distance between atomic particles in the material.
Light radiation carries energy, and the amount of energy carried depends on the square of the amplitude of the wave. In addition, the unit energy depends on the frequency of the wave. This concept of unit (quantum) energy is seldom considered when longer wavelengths are being used, but it determines, to a very considerable extent, what can be done using light waves and particularly the sensing and transducing actions. The quantum nature of light will be explained in more detail when we consider the action of the vacuum photocell, the effect of which was first discovered and explained at the turn of the century. The explanation of photoelectric emission, incidentally, was the achievement for which Albert Einstein won his first Nobel prize.