Colour temperature
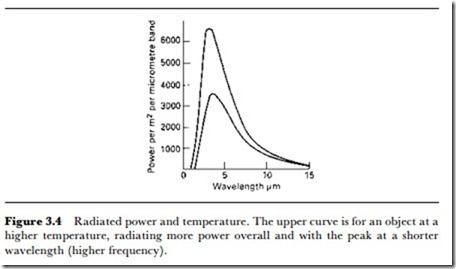
The colour temperature (or equivalent temperature) of light and other radiation is often quoted, and for anyone unfamiliar with the principle it can be very misleading. Any hot object, meaning an object whose temperature is greater than that of absolute zero, will radiate energy, but the spectrum of that energy, meaning the relative percentage of the energy that is radiated at each detectable frequency, will depend on the temperature of the object. When an object and its surroundings are at the same tem- perature, each radiates to the other and the amount of energy leaving the object is balanced by the amount entering, keeping the temperature constant. The radiation is always over a wide band of frequencies, with a definite peak at one frequency. Figure 3.4 shows typical graphs of radiated power density (Wjm2) plotted against wavelength of light. The vertical
axis is scaled in terms of power radiated per m2 of area per unit bandwidth; the horizontal axis is scaled in J.m of wavelength.
Since any object hotter than absolute zero will radiate, the radiation temperature is measured in Kelvins (degrees absolute) which for all practical purposes are calculated by adding 273 to Celsius temperature. At low absolute temperatures, the radiation is predominantly in the far infrared, with no trace of near IR (which would be easier to detect) or visible light. As the temperature is raised, the spectrum shifts towards the higher frequencies and the absolute amount of energy at each frequency increases. In other words, as an object is heated, it radiates very much more energy and the energy is predominantly of a shorter wavelength. The amount of energy radiated is proportional to the fourth power of the absolute temperature, so that an object at 1000K is radiating 16 times as much as one at 500K.
At a temperature of around 700K, the spectrum of radiation has shifted sufficiently for some of the radiation to be in the visible region, so that we say that the object is red-hot. As the temperature increases, the spectrum continues to shift, with the simple relationship that the wavelength of the peak multiplied by the absolute temperature is a constant (double the absolute temperature and you halve the wavelength of the predominant radiation). In addition, the bandwidth of the spectrum increases. Temperatures of 3000K or so will provide light that is predominantly blue, approaching the spectrum of sunlight.
The spectrum of any radiation can therefore be described very compactly in terms of its coLour temperature, meaning the temperature to which a perfectly radiating object (a bLack body) would need to be raised to radiate in the same way. To say that a light has a colour temperature of 3500K does not necessarily mean that it comes from an object at that temperature, only that it has the same composition in terms of predominant frequency and bandwidth as light from an object at that temperature. Fluorescent tubes, for example, can give light of a high colour temperature but are themselves cool to touch. In this example, the discrepancy is due to the small fraction of the contents of the tube that is actually radiating. There will usually be a discrepancy between actual temperature and colour tem- perature for any radiating material.