EXTRA COOLING CAPACITY
In excessively hot climates and/or if the engine is overloaded as by pulling a heavy trailer up long grades, extra cooling system capacity may be re quired. Methods of increasing the cooling capacity are described below. Most car makers offer one of these methods:
1. A slightly thicker radiator core.
2. A fin and tube radiator core with more fins, or a cellular core with more and finer water pas sages.
3. A radiator core made of copper instead of brass because copper is a better conductor of heat.
4. A higher speed fan obtained by reducing the diameter of the fan pulley or increasing the size of the crankshaft pulley.
5. A fan of greater capacity with five blades instead of four, wider blades and/or blades with greater curvature (pitch), or the fan diameter may be increased.
6. Closed cooling systems add capadty by using an auxiliary tank in addition to the usual two radiator tanks. Called expansion, reserve, surge and overflow tanks these devices serve sev eral functions that in effect increase the heat rejection ability of the cooling system.
ANTI-FREEZE COMPOUNDS
The purpose of an anti-freeze compound is to depress the freezing point of the coolant so that it will remain fluid in freezing weather. There are only two anti-freeze compounds in common use, namely, methanol and ethylene glycol. (All anti freeze base compounds should be properly in hibited to prevent rust and corrosion in the cool ing system.)
Methanol is an abbreviation of methyl alcohol which is the chemical name for wood alcohol.
When adding anti-freeze to a car, it is necessary to know what kind of anti-freeze is already in the system. Methanol has a characteristic odor. Ethyl ene glycol has no noticeable odor.
When the word alcohol is used in this discus sion it means methanol.
ANTI-FREEZE DATA
The addition of methanol alcohol or ethylene glycol base anti-freezes to water depresses the freezing point of the coolant. For example, pure ethylen e glycol freezes at _go F. However, as water is added the freezing point is lowered. The maximum freezing protection of about 92° F be low zero is given by a 66% pure ethylene glycol to 34% water solution.
The number of quarts of anti-freeze to use in a cooling system for whatever freezing point is de sired is listed on charts supplied to service stations by the anti-freeze maker. The quantities of anti-
, freeze specified in these charts for prevention of freezing at a given temperatur e mean that no ice crystals will form if the temperature does not go any lower.
For example, a methanol chart will show that three quarts of methanol in an 18-quart cooling system prevents the formation of ice crystals down to 15 degrees. As the temperature drops below 15 degrees, more and more ice crystals are produced, until finally the radiator core and/or lower hose become clogged with slush ice which either greatly reduces the water circulation or stops it entirely; then the water in the water jackets boils. This explains why you see steaming cars in very cold weather.
To elaborate this point, when the engine is idling or the car is driven slowly on a cold day, the ice crystals will accumulate in the lower hose connection and when enough of them have formed the hose will be completely clogged. On the other hand, if the car is allowed to stand with the engine shut off, the ice crystals will form in the passages of the core apd will eventually block it completely.
Slush cannot form if the cooling system contains enough anti-freeze to give full prot ection down to the lowest cold weather temperature anticipated. It is interesting to note, however, that if the cooling system contains 10 per cent or more of anti-freeze neither the cylinder block nor the radi ator will be damaged because you have a mixture which is composed of 90 per cent ice crystals surrounded by 10 per cent liquid anti-freeze. This thick but semi-liquid mixture is unable to produce enough pressure to damage either the engine or the radiator.
COOLING SYSTEM DAMAGE
The regulation of engine operating temperature by the cooling system is indispensable for depend able performance, maximum power and economy of operation.
When a car is new all radiator passages, water jackets, hose and other parts of the cooling system are clean and are able to carry away waste heat efficiently. After awhile, however, if the system is not kept leak-proof and protect ed against corro sion, rust will form in the water jackets of the cylinder block and head and will be carried to the radiator during normal water circulation. This rust acts as a blanket and prevents the water from being properly cooled and restricts its circulation. If allowed to remain, these deposits will eventu ally shut off circulation entirely, resulting in en gine overheating.
Overheating
Overheating not only causes engine knock and loss of power, but also will result in damage to bearings and other moving parts. Cylinder heads and engine blocks are often warped and cracked by terrific strains set up in the overheated metal, especially when cold water is added afterward without allowing the engine to cool.
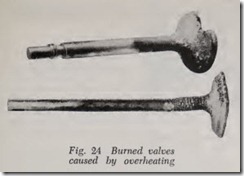
If a car is operated with boiling water, steam pressure forces large quantities of water out of the cooling system through the radiator overflow pipe. More violent boiling occurs, and still more water is lost. Finally, water circulation is stopped and cooling fails completely. This means that operat ing an engine with boiling water for even a short length of time may be actually driving that engine to destruction. Fig. 24 shows a pair of burned valves caused by overheating.
Overcooling
Although less sudden in effect than overheating, overcooling may be equally dangerous to the engine. Low engine operating temperature, espe cially during freezing weather, results in excessive fuel consumption, dilution of engine oil by un burned fuel, and formation of sludge from con densation of water in cylinder and crankcase.
Lubrication failure may follow sludge forma tion and lead to serious engine damage. Burned fuel vapors also mix with water in the crankcase and form corrosive acids which attack engine parts. Fig. 25 are examples of piston and pin damage due to overcooling.
Cooling System Corrosion
A chemical combination of iron, water and air produces rust. The water jacket of the engine has a large mass of iron exposed to the cooling water, and no cooling system is free of air. Thus all elements of rust formation are found in the cool ing system. Over 90 per cent of the solid matter that clogs radiators is rust.
The rate of rust formation in the cooling system is influenced by many conditions of service and operation. Air mixed with water can increase cor rosion of iron as much as 30 times. The normal source of air in the cooling system is the radiator top tank. At high engine speeds, the rush of water into the radiator is great enough to drive air into the liquid and carry air bubbles down through the water tubes in the radiator. If the water level is allowed to drop as low as the top of the tubes, suction of the water pump will draw air into the overflow pipe and down through the tubes.
Heat speeds up corrosion and unfortunately the rate of corrosion with iron appears greatest at water temperature corresponding to best engine performance. Iron, solder and copper will corrode more than twice as fast at 175 degrees than at 70 degrees.
Some waters arc less corrosive to iron than distilled or rain water. But others that contain dissolved mineral salt impurities are particularly harmful to cooling system metals. Any acid condi tion in water will increase iron corrosion and rust f orma tion. Hard water containin g large amo unts of lime and certain other minera ls will deposit scale at ”hot spots” in the engine water jac ket if large quantities of such water are added to the cooling system over a period of time.
The cooling water may become contaminated as a result of extended service, a faulty condition within the system, or from improper maintenance. Excessive aeration from a neglected suct ion leak at the water pump or at any point between the pump and radiator speeds up corrosion and shortens the rust-free life of the coolant. Combus tion gas dissolved in water from a leak at the cylinder head joint has a similar effect to aeration. Corrosive contamination of the water can also result from failure to neutralize and flush out cleaning solution.
Effects of Rust Formation
If rust deposits are allowed to build up in the water passages of the block or head, Fig. 26, they may hold enough heat in the metal to create local ”hot spots”, especially around the valve seats. Steam pressure from local boiling at such places is a hidden although common cause of overflow loss. The metal may get so hot as to cause sticking, warping, or burned valves, or even a cracked block or head.
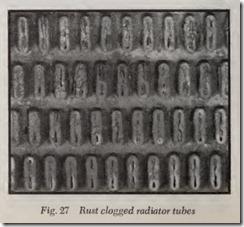
Rust deposits have their most harmful effects in the radiator. Even a small amount of fine rust particles continually circulating through the radi ator has a tendency to spread out in the form of a thin, hard scale on the inside of the narrow water tubes, Fig. 27. This scale first reduces cooling efficiency of the r adiator by insulating the tubes from the water. As more rust becomes lodged in the tubes, circulation is restricted and boiling starts. When boiling starts, large amounts of rust are stirred up in the water jacket and carried over into the radiator. Further operation of the car will result in overheating, loss of power and engine damage.
Corrosion Damage
Although a less common cause of trouble than rust clogging, corrosion damage to metal parts can be equally serious. For example, when a water distributin g tube in the block becomes perforated by corrosion , Fig. 28, wat er distribution in the water j acket is completely upset. Some valves and cylin ders will be robbe d of proper circulation and coolin g, an d hot spots, overheating, sticking valves, and even heat-cracking may follow.
Corrosion prevention is especially important for such parts that are so completely h idden within the engine th at preventive inspection is impracti cal and detection of failure is difficu lt. Among oth er metal parts sometimes damaged by corro sion ar e r adiators, w ater pumps, cylinder heads, Fig. 29, and core hole plugs. Thin m etal parts are weakened by corrosion and crack more easily when subjected to vibration and strain.
Importance of Rust Prevention
A rusty cooling systerp m ay seem to function satisfact orily und er moderate operating conditions but will fail to cool the engine under more severe conditions, often just at the time when full power output is more urgently needed. The system can be kept practically rust-free, and loss of cooling efficiency from rust formation can be avoided by periodic corrosion preventive services.
Protection of the cooling system against rust corrosion is accomplished by adding an inhibitor to the cooling water. These inhibitors are com mercially available, and laboratory tests have shown that a corrosion inhibitor in water reduces the normal rusting of iron at least 95 per cent.
Corrosion protection is particularly important during warm weather driving when water alone is used as a coolant, since there is more air in water and more rusting in the system. In very cold weather, control of coolant circulation by the thermostat may reduce the flow into the radiator to only a few gallons per minute and very little air is driven into the coolant.
In hot weather with thermostat wide open, the How into the radiator at high engine speeds may increase to 100 gallons per minute or more in some engines. The resulting increase in coolant
aeration, together with a higher metal and coolant temperature, greatly speeds up the rate of rusting.
Many anti-freeze compounds contain a corrosion inhibitor. If one of these compounds is in stalled, no inhibitor of any kind should be added. If a cooling system is dirty enough to require cleaning, anti-freeze solutions would surely be contaminated and probably inhibitors depleted. The system should have a fresh fill of anti-freeze or inhibited water.