Acidity/alkalinity
The acidity or alkalinity of water is an important factor for water suppliers, and also for all users of water such as chemical plants, generating stations, agriculture and horticulture. The acidity or alkalinity of water is measured on the pH scale, on which perfectly neutral water has a pH value of 7, fairly strong acid solutions have a pH of 2 or lower and fairly strong alkali solutions have a pH of l2 or more. The basis of this scale is the relative amount of free hydrogen ions in the water, and is outlined in Table 7.6.
The sensing of pH can be fairly simple if all that is needed is an indication of a change in the ionization. Perfectly neutral water, with a pH of 7.00, has a very high resistivity value, but any trace of ionization will cause the resistivity to drop very sharply. Some care is needed in the measurement of resistivity, however, because most metals dissolve to some extent in water, creating ions and causing a resistivity change. Metals such as platinum or palladium are best suited to this type of use, but this type of indication does not show whether the conductivity is due to hydrogen ions (acidity), hydroxyl ions (alkalinity) or metallic ions (contamination).
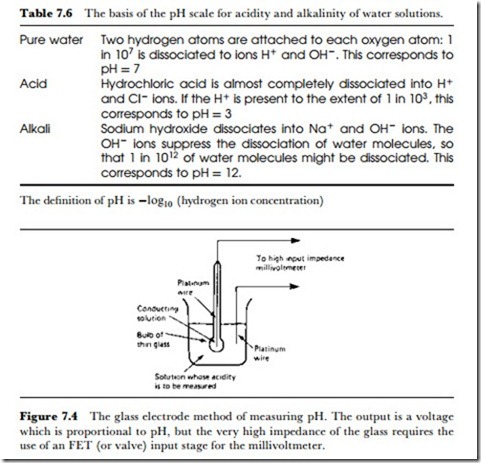
The standard electrical method for reading pH depends on the glass- electrode system, illustrated in Figure 7.4. The glass bulb is very thin and contains a mildly acidic solution which is a good conductor. A platinum
contact is inside the bulb, and another platinum contact is immersed in the water whose pH is being measured. The EMF that exists between the glass electrode and the external platinum wire is then a measure of the pH value in the water. The resistance of this cell is high, of the order of l00 MQ, so that high input-impedance MOS DC amplifiers are needed to read the few mV of output. The glass-electrode pH measuring system is fragile, and the readings are easily upset if the glass is allowed to dry out or become stained, but of the available methods it is the most reliable for any kind of use outside laboratory conditions. Calibration is easily carried out, because solutions of standard and constant pH value (buffered solutions) are easily obtained.