Resistance Losses
The ions travelling through the electrolyte between anode and cathode, and the electrons forming the electrical current that provides electrical power, experience resistance in the media they move in. The overall internal resistance of the fuel cell is denoted by Ri.
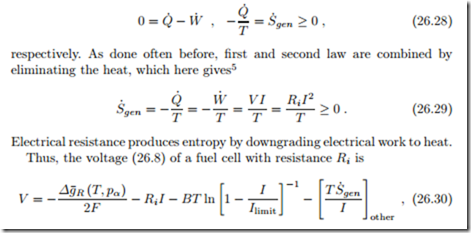
Electrical resistance is an irreversible process, and we proceed by deter- mining the corresponding entropy generation, for a resistor at steady state.
The resistor consumes work in form of electrical power W˙ = −VI = −RiI2, where I is the current and V is the voltage. The temperature of the resistor is T , and first and second law reduce to
where the last term refers to other contributions to entropy generation.
In PEM fuel cells, a particular contribution to resistance loss is the drying-out of the membrane. The protons travelling from anode to cathode drag some water along, and this water is removed together with the product water. Thus, the membrane becomes somewhat dryer at the anode, and this reduces its conductivity, i.e., increases the membrane resistance. A common method to deal with this problem is to moisturize the incoming hydrogen fuel, so that new water is available at the anode.