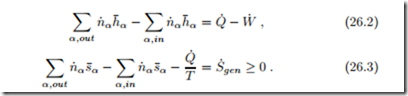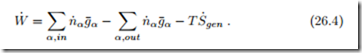Fuel Cell Potential
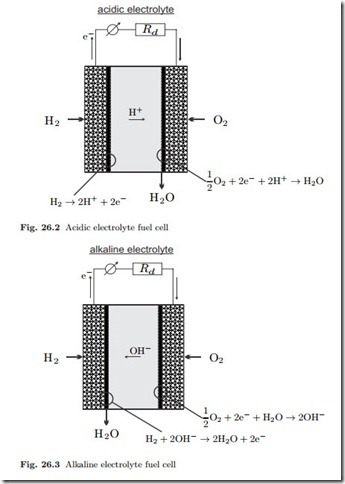
For the thermodynamic analysis of fuel cells, it is not necessary to distinguish between acidic and alkaline fuel cells. We consider the mass and energy flows as in Fig. 26.1, and apply the first and second law, which read for steady state operation
For most of our discussion, the system to be considered is just the fuel cell. For the evaluation we shall assume that all inflows and outflows take place at the homogeneous fuel cell temperature T . Additional irreversible processes (external losses) might occur outside the fuel cell, e.g., in the heating of the incoming oxygen and hydrogen, or in the heat transfer Q˙ cell and its exterior environment. between the fuel Combining the two laws by elimination of the heat Q˙ yields
In order to guarantee sufficient supply of fuel and oxidizer to the gas diffusion layers at all locations along the gas channels, one normally will have excess hydrogen and oxygen which are circulated back to the inlet (at least the hydrogen).
The rate of reactions taking place is denoted by Λ so that the incoming and outgoing mole flows are related to the reaction rate by
For each reaction there are two electrons traveling through the electrical device which corresponds to the electrical current
that the Gibbs free energy of reaction is the maximum amount of work that can be obtained per mole of fuel. This work could be obtained from a fully reversible fuel cell, where Vover = 0.
When the electron circuit is interrupted, reactions cannot take place, and accordingly no irreversible processes occur. In this case one measures the open circuit voltage between anode and cathode,1
At standard reference conditions, the open circuit potential of a hydrogen fuel cell is V0 = 1.23V . Higher voltages, and thus higher powers, are obtained by connecting several cells in series, to form fuel cell stacks.