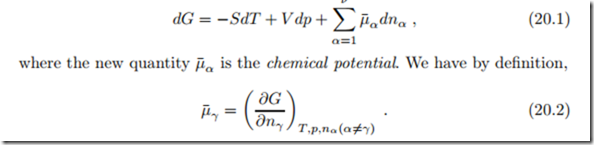Definition and Interpretation
Changes in the composition of the mixture, either by addition or removal of components or by reaction, change the properties of the mixture, in particular the Gibbs free energy. A straightforward extension of the Gibbs equation which accounts for the change of Gibbs free energy with varying composition is
The chemical potential is of fundamental importance in the thermodynamics of inert and reacting mixtures. To understand its physical meaning, we consider two mixtures of different composition and different pressures but equal temperatures T , which are divided by a semi-permeable membrane that only allows component γ to pass, as depicted in Fig. 20.1. Since pressure and temperature are controlled, we know that in equilibrium the Gibbs free energy of the system must assume a minimum,
G −→ Minimum . (20.3)
The total Gibbs free energy of the system is the sum of the Gibbs free energies of the two parts I and II of the system, which depend only on the pressure, temperature and mole numbers within their portion of the system,
Just as the continuity of temperature at diathermic walls allows us to measure temperature, the continuity of the chemical potential at semi-permeable walls allows us its measurement, at least in principle. And just as temperature differences lead to heat flow and allow for power generation, differences in chemical potential cause particle flow and allow for power generation, as will be discussed in Section 20.7.
Nevertheless, the chemical potential is a rather abstract quantity, and we need to study it further, and relate it to quantities we are more familiar with.