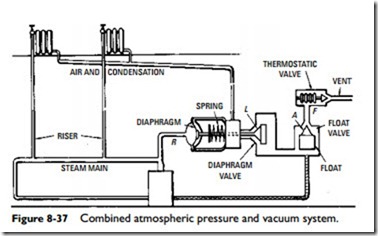
Combined Atmospheric Pressure and Vacuum Systems
A combined atmospheric and vacuum system works at pressures in the boiler from 1 to 5 oz of gauge pressure (that is, above atmospheric). This pressure is needed on coal-burning installations to operate the damper regulator.
The desired vacuum in the heat-emitting units is obtained by throttling the supply steam with the unit feed valves. The working principle of this system is shown in the elementary sketch found in Figure 8-37. In operation, when steam is raised in the boiler, it passes through the steam main risers and supply valves to the heat-emitting units.
The proper working of this system is obtained by an automatic device or trap that closes against the pressures of either steam or condensation and allows air, but not the steam, to pass out. The trap (Figure 8-37) consists of three elements: (1) a diaphragm valve (point L), (2) a float valve (point A), and (3) a thermostatic valve (point F). Connection R, in Figure 8-37, connects the steam outlet of the boiler to the diaphragm. When there is no pressure in the boiler, diaphragm valve L is held closed by the spring.
When the fire in the boiler is started and the air in the boiler expands, the diaphragm is inflated and moves the valve spring to the right (against the action of the spring), which opens valve L. This makes a direct opening through float valve A and thermostatic valve F, thus opening the system to the atmosphere. Valve L remains open as long as there is a fraction of an ounce of pressure on the boiler. When steam forms and passes through the system, it drives all the air out of the system through the three open valves (L, A, and F).
The heat of the steam causes the expansion element of valve F to expand and close the valve; thus the system is filled only with steam.
The vacuum is obtained on the principle that the steam admitted to the radiators condenses while giving off heat through the radiator walls. This causes a tremendous reduction in volume of the steam remaining in the radiators, resulting in a pressure that is less than atmospheric in radiators; i.e., a vacuum is formed. The steam condenses because of a reduction in temperature below that corresponding to the pressure of the steam.
When the radiator gives off heat in heating the room, the temperature of the steam in the radiator is lowered. This reduction in temperature causes some of the steam to condense in a sufficient amount to restore equilibrium between temperature and pressure of the steam.
The pressure falls because the temperature falls. If a closed flask containing steam and water is allowed to stand for a length of time, the atmosphere being at a lower temperature than that inside, the flask will abstract heat from the steam and water, but the heat will leave the steam more quickly than it leaves the water. The result is a continuous condensation of the steam and reevaporation of the water, during which process the temperature of the whole mass and the boiling point are gradually lowered until the temperature inside the flask is the same as that outside. This process is accomplished by a gradual decrease in pressure. Figures 8-38 through 8-40 illustrate the effect of pressure on the boiling point.
A one-pipe system may be converted into a combined system by replacing the air vent on the heat-emitting units and making the piping absolutely tight. In this conversion, a compound gauge recording both pounds of steam and vacuum inches is required (Figure 8-41).