SYSTEM CLEANING TREATMENTS
Air conditioning or refrigeration is basically the controlled removal of heat from a specific area. The refrigerant that carries heat from the cooled space must be cooled before it can be reused. Cooling and condensation of refrigerant require the use of a cooling medium that, in many systems, is water.
There are two types of water-cooled systems. The first type uses once-through operation. The water picks up heat and is then discarded or wasted. In effect, this is 100 percent, or total, bleed. Little if any mineral concentration occurs. The scale that forms is due to the breakdown of bicarbonates by heat. These form carbonates, which are less soluble at high temperatures than at low. Such scale can be prevented through use of a treatment chemical.
The other type of water-cooled system is the type in which heat is removed from water by partial evapo- ration. The water is then recirculated. Water volume lost by evaporation is replaced. This type of system is more economical from the standpoint of water use. However, the concentration of dissolved minerals leads to conditions that, if not controlled and chemically treated, may result in heavy scale formation.
Evaporative Systems
One method of operating evaporative recirculating systems involves 100 percent evaporation of the water with no bleed. This, of course, causes excessive mineral concentration. Without a bleed on the system, wa- ter conditions will soon exceed the capability of any treatment chemical. A second method of operation em- ploys a high bleed rate without chemical treatment. Scale will form and water is wasted. The third method is the reuse of water, with a bleed to control concentration of scale-forming minerals. Thus, by the addition of minimum amounts of chemical treatment, good water economy can be realized. This last approach is the
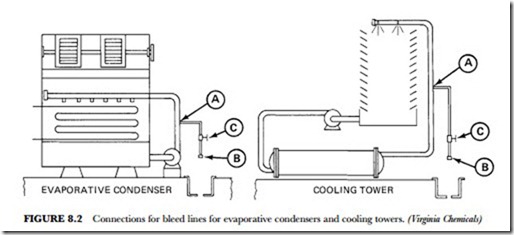
most logical and least expensive. Figure 8.2 shows how connections for bleed lines are made on evaporative condensers and cooling towers.
Scale is formed as a direct result of mineral insolubility. This, in turn, is a direct function of temperature, hardness, alkalinity, pH, and total dissolved solids. Generally speaking, as these factors increase, solubility or stability of scale-forming minerals decreases. Unlike most minerals, scale-forming salts are less soluble at high temperatures. For this reason, scale forms most rapidly on heat-exchanger surfaces.
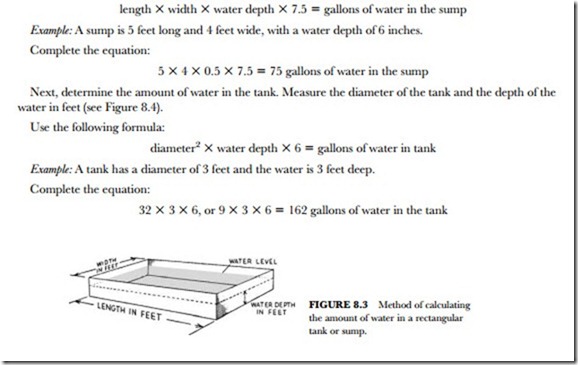
To clean cooling towers and evaporative condensers, first determine the amount of water in the system. This is done by determining the amount of water in the sump. Measure the length, width, and water depth in feet (see Figure 8.3).
Use the following formula:
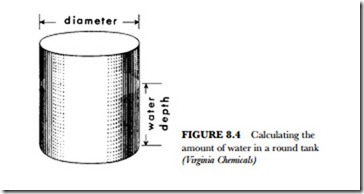
The two formulas above will give you the water volume in either the tank or sump. Each is figured sepa- rately since they are both part of the system’s circulating water supply. There is also water in the connect- ing lines. These lines must be measured for total footage. Once you find the pipe footage connecting the sys- tem, you can figure its volume of water. Simply take 10 percent of the water volume in the sump for each 50 feet of pipe run. This is added to the water in the sump and the water in the tank to find the total system water volume.
For example, a system has 75 gallons of water in the sump and 162 gallons of water in the tank. The sys- tem has 160 feet of pipe:
75 gallons + 162 gallons – 237 gallons
160 feet –: 50 feet = 3.2
75 gallons in sump –: 10 = 7.5 gallons for every 50 feet in the pipes
7.5 gallons X 3.2 = 24 gallons in the total pipe system
237 gallons (in tank and sump) + 24 gallons (in pipes) = 261 gallons in the total system This is the amount of water that must be treated to keep the system operating properly.
Now that you have determined the volume of water in the system, you can calculate the amount of chem- icals needed. To clean the system:
1. Drain the sump. Flush out, or remove manually, all loose sludge and dirt. This is important because they waste the chemicals.
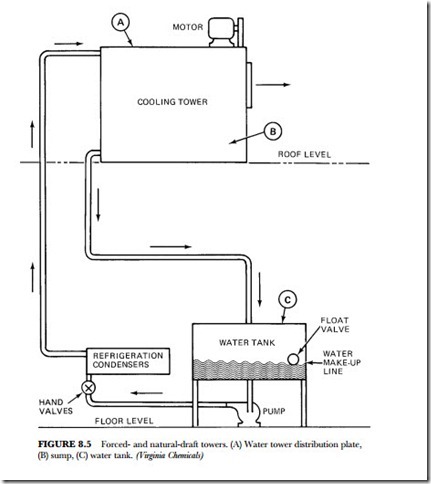
2. Close the bleed line and refill the sump with fresh water to the lowest level at which the circulating pump will operate (see Figure 8.5).
3. Calculate the total gallons of water in the system. Next, while the water is circulating, add starting amounts of either chemical slowly, as follows. (These amounts are for hot water systems.)
For solid scale remover, use 5 pounds per 10 gallons of water. For regular liquid scale remover, use 1 gallon per 15 gallons of water. For concentrated liquid scale remover, use 1 gallon per 20 gallons of water (refer to Figure 8.5). The scale removers can be introduced at the water-tower distribution plate (A), the sump (B), or the water tank (C). Convenience is the keyword here. The preferred addition point is directly into the pump suction area.
4. When using liquid scale remover, add 1 ampoule of antifoam reagent per gallon of chemical.
This will usually prevent excessive foaming if added before the scale remover. Extra antifoam is available in 1-pint bottles. When using the solid scale remover, stir the crystals in a plastic pail or drum until completely dissolved. Then pour slowly as a liquid. Loose crystals, if
(B) sump, (C) water tank. (Virginia Chemicals)
al1owed to fall to the bottom of the sump, will not dissolve without much stirring. If not dissolved, they might damage the bottom of the sump.
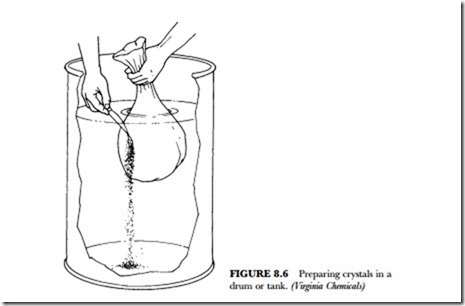
5. Figure 8.6 shows how to prepare the crystals in the drum. Use a 55-gallon drum. Install a drain or spigot about 6 to 8 inches from the bottom. Set the drum in an upright position. Fill the drum with fresh water within 6 inches from the top, preferably warm water at about 80 degrees F (27 degrees C). Since the fine particles of the water-treatment crystals are quite irritating to the nose and eyes, immerse each plastic bag in the water. Cut the bag below the surface of the water. (See Figure 8.6.) Stir the crystals until dissolved. About 6 to 8 pounds of crystals will dissolve in each gallon of water.
6. Drain or pump this strong solution into the system. Repeat this procedure until the required weight of the chemical has been added in concentrated solution. Then add fresh water to fill the system. The treatment should be repeated once each year for best results.
If makeup water is needed during the year, be sure to treat this water also at the rate of 6 pounds per 100 gallons of water added.
Chilled Water Systems
For chilled water systems, follow the instructions just outlined for hot water systems. However, use only 3 pounds of circulating water-treatment solution for each 100 gallons of water in the system. This treatment solution should be compatible with antifreeze solutions.
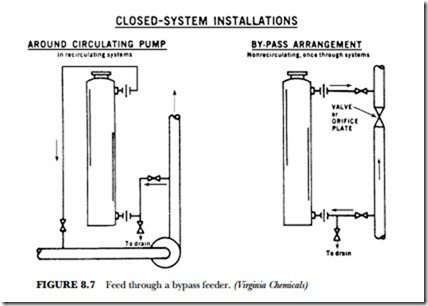
For easy feeding of initial and repeat doses of water treatment solution, install a crystal feeder in a bypass line. The crystals will dissolve as water flows through the feeder. (See Figure 8.7.)
Install the feeder in either a bypass or in-line arrangement, depending upon the application. Place the feeder on a solid, level floor or foundation. Connection with standard pipe unions is recommended.
Connecting pipe threads should be carefully cut and cleaned to remove all burrs or metal fragments. Ap- ply a good grade of pipe dope. Use the dope liberally.
Always install valves in the inlet and the outlet lines. Install a drain line with the valve in the bottom (in- let) line.
Before opening the feeder, always close the inlet and the outlet valves. Open the drain valve to relieve the pressure and drain as much water as necessary. When adding crystals or chemicals molded in shapes (balls, briquettes, etc.), it is advisable to have the feeder about one-half filled with water.
If stirring in the feeder is necessary, use only a soft wood. Stir gently to avoid damaging the epoxy lining.
Fill the feeder to the level above the outlet line. Coat the top opening, the gasket, and the locking grooves of the cap with petroleum jelly or a heavier lubricant.
Open the outlet valve fully. Then slowly open the inlet valve. If throttled flow is desired for control of treatment feed rate, throttle with inlet valve only.
Once the chemicals have been properly introduced, operate the system in the normal manner. Check the scale-remover strength in the sump by observing the color of the solution when using the solid scale remover or using test papers. When chemical removers are used, a green solution indicates a very strong cleaner. A blue solution indicates normal cleaning strength. A purple solution indicates more cleaner is needed. If necessary, dip a sample of the sump solution in a glass to aid color check. Check with the maker of the chemicals and their suggested color chart for accurate work.
If, for instance, Virginia Chemicals scale remover is used in either solid or liquid form, use test papers to check for proper mixture and solution strength. Red test paper indicates there is enough cleaner. Inspection of the evaporative condenser tubes or lowering of head pressure to normal will indicate when the unit is clean. With shell and tube condensers, inspection of the inside of the water outlet pipe of the condenser will indicate the amount of scale in the unit.
After scale removal is completed, drain the spent solution to the sewer. Thoroughly rinse out the system with at least two fillings of water. Do not drain spent solutions to lawns or near valuable plants. The solution will cause plant damage, just as will any other strong salt solution. Do not drain to a septic tank. Refill the sump with fresh water and resume normal operation.
Shell (Tube or Coil) Condensers
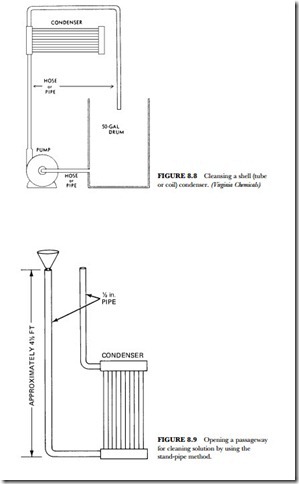
Isolate the condenser to be cleaned from the cooling-tower system by an appropriate valve arrangement or by disconnecting the condenser piping. Pump in at the lowest point of the condenser. Venting the high points with tubing returning to the solution drum is necessary in some units to assure complete liquid filling of the waterside.
As shown in Figure 8.8, start circulating from a plastic pail or drum the minimum volume of water necessary to maintain circulation. After adding antifoam reagent or solid scale remover, slowly add liquid scale remover until the test strips indicate the proper strength for cleaning. Test frequently and observe the sputtering in the foam caused by carbon dioxide in the return line. Add scale remover as necessary to maintain strength. Never add more than 1 pound of solid scale remover per gallon of solution. Most condensers with moderate amounts of carbonate scale can be cleaned in about one hour. Circulation for 30 to 40 minutes without having to add cleaner to maintain cleaning strength usually indicates that action has stopped and that the condenser is clean. Empty and flush the condenser after cleaning with at least two complete fillings of water. Reconnect the condenser in the line.
If a condenser is completely clogged with scale, it is sometimes possible to open a passageway for the cleaning solution by using the standpipe method, as shown in Figure 8.9. Enough liquid scale remover is mixed with an equal volume of water to fill the two vertical pipes to a level slightly above the condenser. Some foaming will result from the action of the cleaner solution on the scale. Thus, some protective meas-
ures should be taken to prevent foam from injuring surrounding objects. The antifoam reagent supplied with each package will help control this nuisance. When the cleaning operation has been completed, drain the spent solution to the sewer. Rinse the condenser with at least two fillings of fresh water.