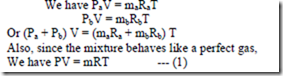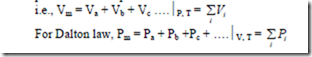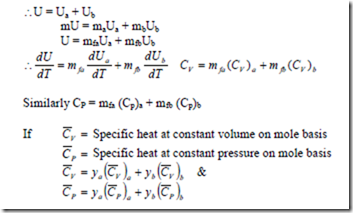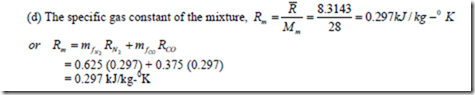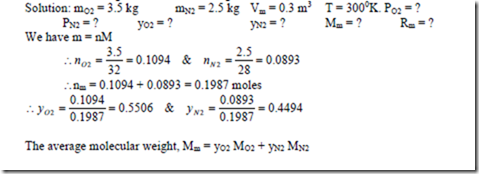Gas constant for the mixture:
By Dalton’s law of partial pressure, which states that, the pressure of mixture of gas is equal to the sum of the partial pressures of the individual components, if each component is considered to exist alone at the temperature and volume of the mixture.
The Amagat-Leduc Law: Expresses the law of additive volume which states that the volume of a mixture of gases is equal to the sum of the volumes of the individual components at the pressure and temperature of the mixture.
Gibb’s Law: It states that the internal energy, the enthalpy and the entropy of a mixture of gas is equal to sum of the internal energies, the enthalpies and entropies respectively of the individual gases evaluated at mixture temperature and pressure.
Isentropic process of gaseous mixture: When a mixture of say two gases, a & b, is compressed or expanded isentropically, the entropy of the mixture remains constant i.e., there is no change in the entropy of the entire system![]()
But this does not mean that there is no change in the entropy of an individual gas. During the reversible adiabatic compression or expansion process, the entropy of one of the two gases will increase, while the entropy of the other one will decrease by the same amount, and thus, as a whole, the entropy of the system will remain constant.
The compression or expansion of each constituent will be reversible, but not adiabatic and hence the energy transferred as heat from one of the two gases must be exactly equal to the energy received by the other one. This is also true when more than two gases are involved in the process.
Volumetric and Gravimetric Analysis: When the analysis of a gaseous mixture is based on the measurement of volume, it is called a volumetric analysis, whereas when it is based on the measurement of mass, it is called the gravimetric analysis. Flue gases generally contain CO2, CO, N2 O2 and H2O in the form of vapour.
The volumetric analysis of a dry flue gas is generally done with Orsat apparatus, which is designed to absorb CO2, O2 and CO. The N2 content of the gas is obtained by difference.
Note 1: The volume fraction & mole fraction of each individual gas are equal. This enables the conversion of the volumetric analysis to gravimetric analysis and vice versa.
Note 2: Molecular weight of common gases is given in Table C-6.
Note 3: Specific heat of gases at constant pressure CP are given in Table C-11
Problems:
1. A perfect gas mixture consists of 2.5 kg of N2 and 1.5 kg of CO at a pressure of 2 bar and at a temperature of 150C. Determine (a) The mass and mole fraction of each constituent, (b) The equivalent molecular weight of the mixture, (c) The partial pressure of each gas, and (d) The specific gas constant of the mixture.
Solution: (a) The total mass of the mixture mm = 2.5 + 1.5 = 4 kg
2. A mixture of gas has the following volumetric analysis. O2 = 30%, CO2 = 40%, N2 = 30%.
Determine (a) the analysis on a mass basis (b) the partial pressure of each component if the total pressure is 100 kPa and a temperature is 320C (c) the molecular weight of the mixture.
3. A mixture of perfect gas at 200C, has the following composition by volume, N2 55%, O2 20%, methane 25%. If the partial pressure of methane is 0.5 bar, determine (i) partial pressure of N2 & O2, (ii) mass fraction of individual gases, (iii) gas constant for the mixture
(iv) molecular weight of the mixture.
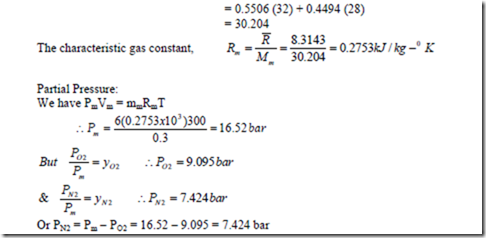
4. A mixture of 3.5 kg of O2 & 2.5 kg N2 is stored in a vessel of 0.3 m3 at a temperature of 270C. Find the partial pressures and mole fraction of each constituent. Also determine the molecular weight and characteristic gas constant for the mixture.
5. A mixture of ideal gases consists of 3 kg of N2 and 5 kg of CO2 at a pressure of 300 kPa and a temperature of 200C, determine (i) the mole fraction of each constituent (ii) molecular weight of the mixture (iii) gas constant of the mixture (iv) the partial pressure and partial volumes of the constituent.
6. A gaseous mixture contains 21% by volume N2, 50% by volume of H2 and 29% by volume of CO2. Calculate (i) the molecular weight of the mixture (ii) gas constant of the mixture (iii) the ratio of specific heats of the mixture. Assume that CP for N2, H2 and CO2 as 1.038,
14.235 and 0.821 kJ/kg-0K respectively.
Related posts:
Incoming search terms:
- what is the characteristic gas constant of methane
- equivalent gas constant
- molecular weight of gas components
- volumetric and gravimetric analysis of gas mixtures
- how to calculate gas constant of a mixture
- gas constant of a mixture
- gas constant
- gas mixture molecular weight vol%
- partial pressure mole fraction
- A mixture of ideal gas consists of 3kg of N2 and 5kg of CO2at a pressure of 300kPa and at 20C
- problems on orsat analysis
- how to find gas constant of a mixture
- how to determine the entropy of mixture of gas
- how to calculate the gas constant of mixture
- to calculate gas constant of mixture
- how to calculate heat of formation of a mixture of gases
- volumetric and gravimetric analysis of gas mixture
- hiw find characteristic gas constant of mixture of gasses
- how to find the gas constant of mixture
- the weight of gases
- real gas constant
- obtaining the adiabatic constant of a gas mixture
- specific volume for ideal gas mixture
- molar fraction of 3kg N2
- tahe gas constnt of the mixture what is the gas constant of components
- mixture of all gases constiturnt system
- mixture gas constant
- how to get gas constant of a mixture
- mixtures of gases volumetric and gravemetric analysis
- gas constant of mixture of gas
- co2 gas constant
- characteristic gas constant of a moxture
- characteristic gas constant
- calculating gas constant of a mixture
- apparent gas constant
- amagat-leduc law
- a rigid tank containig 3kg of gaseous mixture of N2 and Co2 each 50% by volume at 250kN/m2 and 60c receives 1 kg more N2 with the temp remaining 60c for the resulting mixture determine the gravimetric and volumetric analysis
- a mixture of methane and other gases is
- a gaseous mixture contains 55% N2 20% O2 and 25% CO2
- Consider 0 1 kg of an ideal gas mixture that is expanded from 5 bar 1500 K to 1 bar 1100 K The gas mixture has a composition on a molar basis of 22% CO2 3% CO and 75% N2 During this expansion process there is a heat transfer out of 9 kJ across the boundar
- consonants mixture for gas
- gas constant of mixture based on mass fraction
- gas constant of in gas mixture compression
- gas constant mixtures formula
- gas constant methane calc
- gas constant for gas mixture
- formula for gas constant mixture with mole fraction
- equivalent characteristic gas constant
- derive expression to determine gas constant on mixture of gases
- conversion of volumetric analysis to mass analysis