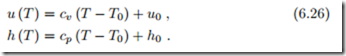Monatomic Gases (Noble Gases)
For monatomic gases, i.e., the noble gases helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn), the specific heats are true constants with the values
and the caloric equation of state follows from straightforward integration as
With cp = const, the integration in (6.20) can be performed easily, and the entropy becomes
Since the resulting expressions for the thermodynamic quantities of monatomic gases are rather simple, these are typically not tabulated.
Related posts:
Commissioning of refrigerating systems:Refrigerant recovery system
Fireplaces, Stoves, and Chimneys:Chimney Downdraft
Hydronic Heating Systems:Gravity Hot-Water Heating Systems
Hydronic Heating Systems:Hot-Water Boilers
Other Automatic Controls:Potential Relay
Air-Conditioning:The Comfort Chart and Cooling Load Estimate Form
Examples on Heat Exchangers
Hydronic Heating Systems:Hydronic Furnaces
Oil Furnaces:Chimneys and Chimney Troubleshooting