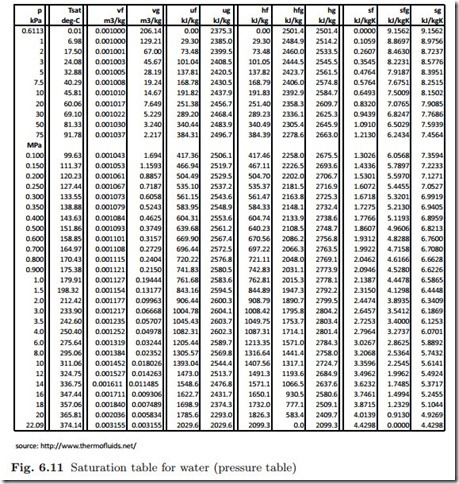
Identifying States
Quality can only have values between 0 and 1. If one finds values outside this range, one either has compressed liquid, or superheated vapor.
A state of given temperature T for which another property (v or u or h or s) is known, is compressed liquid for
A state of given pressure p for which another property (v or u or h or s) is known, is compressed liquid for
and it is superheated vapor if
It is a useful exercise to verify the above conditions by means of p-v-, T-s-, and p-T-diagrams!
Related posts:
Oil-Fired Furnaces – AIR FILTER
Crazy Gay-Lussac’s Gas Expansion Emporium
HEAT SOURCES:STEAM AND HOT-WATER HEATING
MOTORS:SYNCHRONOUS SPEED
Gas Burners:Venting and Ventilation
Properties and Property Relations:Superheated Vapor
Wind Turbines:Annual energy output and Statistical analysis of wind data Basic equations.
Activation of Reactions:Reaction Rates and the Chemical Constant.
The First Law of Thermodynamics:Internal Energy and the Caloric Equation of State