Problems
Enthalpy of Formation and Heat of Reaction
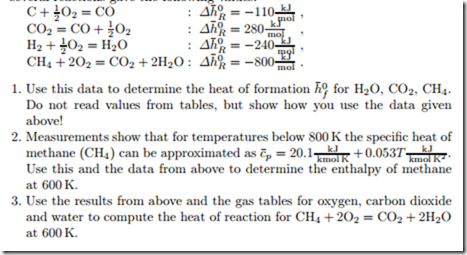
Measurement of the heat of reaction at standard reference state T0, p0 for several reactions gave the following values:
Dimethyl Ether (DME)
The heat of reaction at standard conditions for the combustion of gaseous dimethyl ether, C2H6O, is experimentally found as −1460 kJ , when the product water is liquid.
1. Use this measurement and tabled data to determine the enthalpy of formation of DME.
2. Determine the heat of reaction when the product water is vapor.
Shifting the Chemical Equilibrium
Consider a mixture of CO2, CO and O2 in chemical equilibrium. Now the pressure is doubled. Will the number of moles of CO2, CO and O2 change? How? How does the equilibrium change when the temperature is increased?
Changes in Chemical Equilibrium
An equimolar mixture of CO and H2O(g) reacts to form an equilibrium mixture of CO2, CO, H2O and H2 at 1727 ◦C, 1 atm.
1. Will decreasing the pressure while keeping the temperature constant in- crease or decrease the amount of H2 present? Explain.
2. Will lowering the temperature increase or decrease the amount of H2 present? Explain.
Shift in Chemical Equilibrium through Inert Addition
An equimolar mixture of O2 and H2 reacts to form an equilibrium mixture of O2, H2, and H2O. After equilibrium is reached, N2 is added to the mixture isobarically. As nitrogen is added, does the amount of water increase or decrease? Use Le Chatelier’s principle for a first answer. Then perform a detailed analysis to find a relation between the amounts of N2 added and H2O present (assume stoichiometric mix of H2 and O2).
Shift in Chemical Equilibrium
Methane, CH4, reacts with stoichiometric air to form an equilibrium mixture of CH4, CO2, H2O, O2, N2. Will the equilibrium between CH4, CO2, H2O, O2 be different when the reaction takes place at the same temperature and pressure, but no nitrogen is present? State your arguments, e.g., consider the quotient pCH4 /pCO2 .
Dissociation of Oxygen
Measurement of oxygen at 3800 K shows equal mole fractions of O2and O.
1. Determine the pressure.
2. Now the pressure is doubled. Determine the mole fractions of O2 and O.
Law of Mass Action: Methanol Synthesis
Methanol (CH3OH) is produced by catalytic hydrogenation of carbon monox- ide according to the reaction
Assume that all partners in the reaction are ideal gases.
1. Carbon monoxide and hydrogen are mixed in stoichiometric ratio. Find an expression that relates the chemical constant Kp (T ), the methanol mol ratio XCH3 OH and the total pressure p of the mixture.
2. A measurement at 600 K shows that Kp (600 K) = 12000 bar2. For which total pressure p do we have a methanol ratio of 30%?
3. The reaction is exothermal. What does that mean? Would a further increase of temperature increase the methanol ratio?
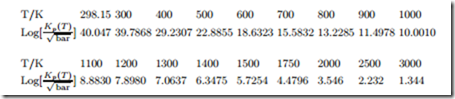
Law of Mass Action: Formation of NO
Air (79% N2 and 21% O2) is heated to 2000 K at a constant pressure of 2 bar. Assume that the equilibrium mixture at this temperature consists of N2, O2, and NO.
![]()
![]() For the reaction equation 1 N2 + 1 O2 = NO one finds at this temperature that ln Kp(T ) = −3.931.
For the reaction equation 1 N2 + 1 O2 = NO one finds at this temperature that ln Kp(T ) = −3.931.
1. Compute the mole fractions of the three components in equilibrium.
2. Will the equilibrium composition change when the pressure is doubled?
3. For this reaction, ln Kp grows with temperature. Does that mean the reaction is exothermic or endothermic?
Law of Mass Action: N2O4
One kmol of N2O4 dissociates at 25 ◦C, 1 atm to form an equilibrium ideal gas mixture of N2O4 and NO2, in which the amount of N2O4 is 0.8154 kmol.
1. Determine the mole number of NO2 in equilibrium.
2. Determine the chemical constant Kp (T ) at 25 ◦C for the reaction.
3. Determine the amount of N2O4 that would be present if the pressure is 0.5 bar.
Hint: Determine first the absolute mole numbers of NO2 and N2O4.
Chemical Reaction
1. Compute the mass flow of oxygen.
2. The partial pressure of water vapor in the exhaust should be larger than 95% What is the maximum temperature for the combustion chamber, and what heat flux must be removed? Repeat for water conent of 98%.
3. Discuss the flame temperature under adiabatic conditions. Derive an equation that contains only Tflame as the unknown (or a set of equations, which will serve for the same purpose) – that equation will contain Kp(T ), hα(T ) for all components).
Steam Methane Reforming
Steam methane reforming is used to produce hydrogen from methane, in particular for further use in ammonia production. The first reaction step in steam methane reforming is the reaction
which is then followed by the water gas shift reaction discussed in the next two problems.
At 1173 K, the chemical constant for this reaction is Kp (1173 K) = 1.43 × 103 bar2. Methane and steam are mixed in the ratio 1 to α by mole, and react with help of catalysts at a pressure of 30 bar.
Find the equations to determine the partial pressures pCH4 , pH2 O, pH2 , pCO. Use a computer to determine the mole fractions of all components in chemical equilibrium at the given pressure and temperature for α = 1 and α = 5. Also determine as a measure for the relative conversion of methane into hydrogen the ratio pCH4 /pH2 . Interpret the result–can you explain it?
Assume that all components behave as ideal gases.
Property data: Water Gas Shift Reaction I
The water gas shift reaction is the second reaction for the production of hydrogen from methane. The reaction equation reads
1. Assuming that all components are ideal gases, determine the heat of re- action for the temperature T = 600 K. Is the reaction exothermic or endothermic?
2. Determine the Gibbs free energy of reaction at T = 600 K and p = 1 atm.
3. Compute the chemical constant KX (T, p). Note: this asks for KX , not for Kp.
4. For this reaction, does the Gibbs free energy of reaction depend on pressure? Explain! Determine Kp (T ).
Law of Mass Action: Water Gas Shift Reaction II
Consider the chemical equilibrium of carbon monoxide, carbon dioxide, water and hydrogen through the water gas shift reaction, as above, at T = 800 K and p = 1 atm. The chemical constant for this reaction is KX (800 K, 1 atm) = 16.424.
1. Determine the equilibrium mole fractions for all components, when the initial state was a stoichiometric mixture of carbon monoxide and water vapor.
2. Can the equilibrium be shifted by a change of pressure? Explain!
Law of Mass Action: High Temperature Combustion
Reaction of a stoichiometric mixture of benzene (C6H6) and dry air at 200 kPa, with a small heat loss, results in a flame temperature of 2400 K. Assume that nitrogen, oxygen and water do not dissociate, and consider the equilibrium between CO2, COand O2. Determine the partial pressures of all components in the mixture.
Law of Mass Action: Incomplete Combustion
Octane (C8H18) is burned with 95% theoretical air. The resulting equilibrium mixture consists of CO2, CO, H2O, H2 and N2. Determine the mole fractions of all constituents when the mixture is at 1000 K and 2 bar.
Law of Mass Action: Methane
At 3000 K and 8 bar, methane (CH4) reacts with the stoichiometric amount of pure oxygen according to CH4 +2O2 ↔ CO2 +2H2O. The chemical constants for the following reactions are given (all at 3000 K):
Determine the mole fraction of CH4 in equilibrium.
Law of Mass Action with Multiple Reactions
A mixture consists originally of 3 kmol of CO2 and 1 kmol of O2. Assume that in equilibrium at 3000 K and 3 bar only CO2, CO, O2 and O are present, and determine the equilibrium composition.
Law of Mass Action: Nitrogen and Oxygen
A gas mixture consisting of 1 kmol of NO, 10 kmol of O2 and 40 kmol of N2 reacts to form an equilibrium mixture of N2, NO2, NO, and O2 at 500 K, 0.1 atm. Determine the composition of the equilibrium mixture.
1. Solve for the case that the molecular nitrogen stays inert.
2. Solve for the case that the molecular nitrogen reacts.