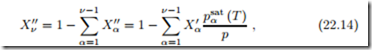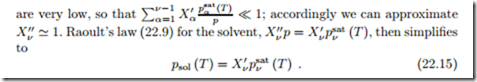Saturation Pressure and Temperature of a Solvent
We consider solutions of low volatility components, e.g., salts, in a more volatile solvent, e.g., water, in liquid-vapor equilibrium. The mole fraction of solvent vapor is
where we used Raoult’s law for the mole fractions of the dissolved compo- nents, X //. For the non-volatile substances the saturation pressures psat (T )
Here, psol (T ) denotes the actual saturation pressure of the solvent vapor over the solution at temperature T . In other words, the ideal mixture of composition X at temperature T will boil when the pressure is psol (T ).
According to (22.15) the actual pressure in the solvent vapor, psol , is smaller than the saturation pressure of the solvent alone, psat (T ), that is the dissolved substances reduce the volatility of the solvent. The reason for this is a competition between solvent vapor and the salt dissolved in the liquid to increase their entropy: The solvent vapor has a larger entropy than the solvent liquid, but the dissolved salt has a larger entropy when it can access a larger volume, that is when there is more liquid solvent.
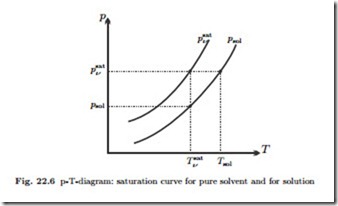
Figure 22.6 shows the saturation curves for the pure solvent and the so- lution in a p-T-diagram. The curve for the pure solvent (psat) lies above the curve for the solution (psol), in accordance with (22.15). The figure shows that for a given pressure p the saturation temperature Tsol (p) of the solution is higher than the saturation temperature T sat (p) of the solvent alone: at a given pressure saltwater boils at higher temperatures than pure water. To estimate the change in saturation temperature, we assume that the slope of the new saturation curve is close to the slope of the saturation curve of the solvent as described by the Clausius Clapeyron equation (17.46), that is
With the assumptions that the liquid volume can be neglected against the vapor volume, and that the vapor follows the ideal gas law, and with (22.15), this yields
Tsol is the temperature at which an ideal mixture of composition X/ at pressure p will boil.