Phase Mixtures
In this chapter we discuss equilibria between mixtures in different phases, e.g. liquid-vapor equilibria, or liquid-solid equilibria. Phase mixtures normally are characterized by different mole fractions (or concentrations) in the different phases. We shall discuss phase diagrams, changes of melting and evaporation temperatures in solutions, distillation processes, and gas solubility.
Gibbs’ Phase Rule
For each phase, the mole fractions sum to unity, that is
It follows that a mixture with ν components in f phases is characterized by (ν − 1) f independent mole fractions. In addition, pressure p and temperature T are variables, so that the mixture is characterized by (ν − 1) f + 2 intensive variables, {p, T , X j .
Phase boundaries are permeable interfaces between phases that allow all components to pass, and thus in equilibrium the chemical potentials of all components must be continuous between any two phases,
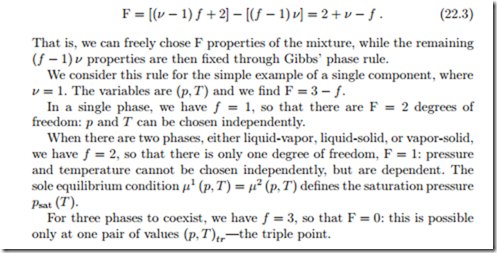
These equations are known as Gibbs’ phase rule, and they give (f − 1) ν conditions1 that restrict the (ν − 1) f + 2 variables. The difference between these numbers gives the number of degrees of freedom for the system,
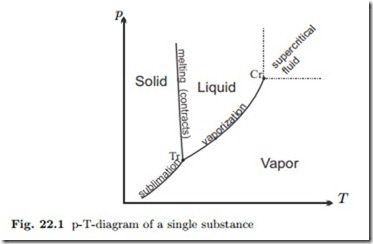
Figure 22.1 shows again a sketch of the p-T-diagram of a single substance (for water, actually) with the saturation curves and the triple point.
Liquid-Vapor-Mixtures: Idealized Raoult’s Law
We study vapor-liquid equilibrium in mixtures where we denote the liquid phase by / and the vapor phase by //. The Gibbs phase rule for this case reads
Our goal is to simplify the phase rule by using constitutive equations for incompressible liquids and ideal gases, respectively. Deviations from this idealized behavior will be studied later. In particular, we make the following assumptions:
1. The liquids are ideal mixtures, so that
When we use the four assumptions in (22.4) together with the defini- tion of the saturation pressure of the single components, g¯/ (T, psat (T )) = α (T, pα (T )), we find Raoult’s law for ideal mixtures (Franc¸ois-Marie Raoult, 1830-1901)
The partial pressure of α in the vapor is given by pα = X //p. Raoult’s law states that the amount of component α in the liquid is proportional to its partial pressure in the vapor.