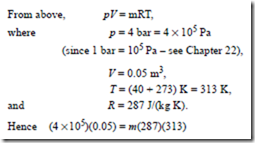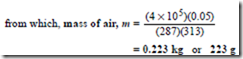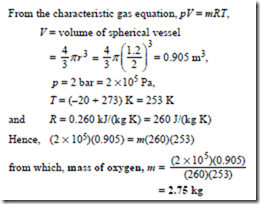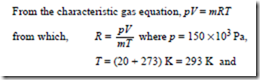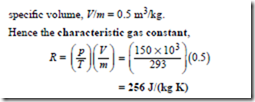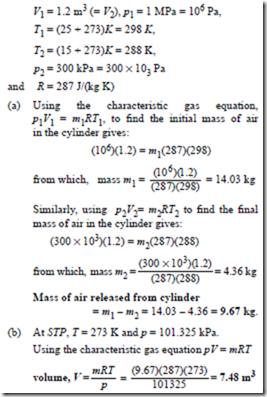Worked problems on the characteristic gas equation
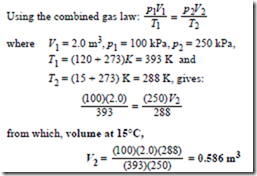
Problem 8. A gas occupies a volume of 2.0 m3 when at a pressure of 100 kPa and a temperature of 120°C. Determine the volume of the gas at 15°C if the pressure is increased to 250 kPa.
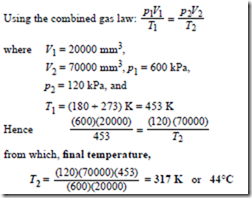
Problem 9. 20000 mm3 of air initially at a pressure of 600 kPa and temperature 180°C is expanded to a volume of 70000 mm3 at a pressure of 120 kPa. Determine the final temperature of the air, assuming no losses during the process.
Problem 10. Some air at a temperature of 40°C and pressure 4 bar occupies a volume of 0.05 m3. Determine the mass of the air assuming the characteristic gas constant for air to be 287 J/(kg K).
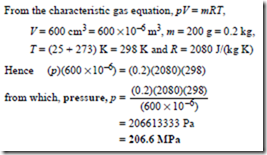
Problem 11. A cylinder of helium has a volume of 600 cm3. The cylinder contains 200 g of helium at a temperature of 25°C. Determine the pressure of the helium if the characteristic gas constant for helium is 2080 J/(kg K).
Problem 12. A spherical vessel has a diameter of 1.2 m and contains oxygen at a pressure of 2 bar and a temperature of –20°C. Determine the mass of oxygen in the vessel. Take the characteristic gas constant for oxygen to be 0.260 kJ/(kg K).
Problem 13. Determine the characteristic gas constant of a gas which has a specific volume of 0.5 m3/kg at a temperature of 20°C and pressure 150 kPa.
Practise Exercise 133 Further problems on the characteristic gas equation
1. A gas occupies a volume of 1.20 m3 when at a pressure of 120 kPa and a temperature of 90°C. Determine the volume of the gas at 20°C if the pressure is increased to 320 kPa.
[0.363 m3]
2. A given mass of air occupies a volume of
0.5 m3 at a pressure of 500 kPa and a tem- perature of 20°C. Find the volume of the air at STP. [2.30 m3]
3. A spherical vessel has a diameter of 2.0 m and contains hydrogen at a pressure of 300 kPa and a temperature of –30°C. De- termine the mass of hydrogen in the vessel. Assume the characteristic gas constant R for hydrogen is 4160 J/(kg K). [1.24 kg]
4. A cylinder 200 mm in diameter and 1.5 m long contains oxygen at a pressure of 2 MPa and a temperature of 20°C. Determine the mass of oxygen in the cylinder. Assume the characteristic gas constant for oxygen is 260 J/(kg K). [1.24 kg]
5. A gas is pumped into an empty cylinder of volume 0.1 m3 until the pressure is 5 MPa. The temperature of the gas is 40°C. If the cylinder mass increases by 5.32 kg when the gas has been added, determine the value of the characteristic gas constant.
[300 J/(kg K)]
6. The mass of a gas is 1.2 kg and it occupies a volume of 13.45 m3 at STP. Determine its characteristic gas constant. [4160 J/(kg K)]
7. 30 cm3 of air initially at a pressure of 500 kPa and temperature 150°C is expanded to a volume of 100 cm3 at a pressure of 200 kPa. Determine the final temperature of the air, assuming no losses during the process. [291°C]
8. A quantity of gas in a cylinder occupies a volume of 0.05 m3 at a pressure of 400 kPa and a temperature of 27°C. It is compressed according to Boyle’s law until its pressure is 1 MPa, and then expanded according to Charles’ law until its volume is 0.03 m3. Determine the final temperature of the gas. [177°C]
9. Some air at a temperature of 35°C and pressure 2 bar occupies a volume of 0.08 m3. Determine the mass of the air assuming the characteristic gas constant for air to be 287 J/ (kg K). (1 bar = 105Pa) [0.181 kg] 10. Determine the characteristic gas constant R of a gas that has a specific volume of 0.267 m3/kg at a temperature of 17°C and pressure 200 kPa. [184 J/(kg K)]
Further worked problems on the characteristic gas equation
Problem 14. A vessel has a volume of 0.80 m3 and contains a mixture of helium and hydrogen at a pressure of 450 kPa and a temperature of 17°C. If the mass of helium present is 0.40 kg determine (a) the partial pressure of each gas, and (b) the mass of hydrogen present. Assume the characteristic gas constant for helium to be 2080 J/(kg K) and for hydrogen 4160 J/(kg K).
Problem 15. A compressed air cylinder has a volume of 1.2 m3 and contains air at a pressure of 1 MPa and a temperature of 25°C. Air is released from the cylinder until the pressure falls to 300 kPa and the temperature is 15°C. Determine (a) the mass of air released from the container, and (b) the volume it would occupy at STP. Assume the characteristic gas constant for air to be 287 J/(kg K).
Problem 16. A vessel X contains gas at a pressure of 750 kPa at a temperature of 27°C. It is connected via a valve to vessel Y that is filled with a similar gas at a pressure of 1.2 MPa and a temperature of 27°C. The volume of vessel X is 2.0 m3 and that of vessel Y is 3.0 m3. Determine the final pressure at 27°C when the valve is opened and the gases are allowed to mix. Assume R for the gas to be 300 J/(kg K).
Practise Exercise 134 Further questions on ideal gas laws
1. A vessel P contains gas at a pressure of 800 kPa at a temperature of 25°C. It is connected via a valve to vessel Q that is filled with similar gas at a pressure of 1.5 MPa
2. A vessel contains 4 kg of air at a pressure of 600 kPa and a temperature of 40°C. The vessel is connected to another by a short pipe and the air exhausts into it. The final pres- sure in both vessels is 250 kPa and the tem- perature in both is 15°C. If the pressure in the second vessel before the air entered was zero, determine the volume of each vessel. Assume R for air is 287 J/(kg K).
[0.60 m3, 0.72 m3]
3. A vessel has a volume of 0.75 m3 and con- tains a mixture of air and carbon dioxide at a pressure of 200 kPa and a temperature of 27°C. If the mass of air present is 0.5 kg determine (a) the partial pressure of each gas and (b) the mass of carbon dioxide. As- sume the characteristic gas constant for air to be 287 J/(kg K) and for carbon dioxide 184 J/(kg K).
[(a) 57.4 kPa, 142.6 kPa (b) 1.94 kg]
4. A mass of gas occupies a volume of 0.02 m3 when its pressure is 150 kPa and its tempera- ture is 17°C. If the gas is compressed until its pressure is 500 kPa and its temperature is 57°C, determine (a) the volume it will oc- cupy and (b) its mass, if the characteristic gas constant for the gas is 205 J/(kg K).
[(a) 0.0068 m3 (b) 0.050 kg]
5. A compressed air cylinder has a volume of 0.6 m3 and contains air at a pressure of 1.2 MPa absolute and a temperature of 37°C. After use the pressure is 800 kPa absolute and the temperature is 17°C. Calculate (a) the mass of air removed from the cylinder, and (b) the volume the mass of air removed would occupy at STP conditions. Take R for air as 287 J/(kg K) and atmospheric pressure as 100 kPa.
[(a) 2.33 kg (b) 1.83 m3]
Practise Exercise 135 Short-answer questions on ideal gas laws
1. State Boyle’s law.
2. State Charles’ law.
3. State the Pressure law.
4. State Dalton’s law of partial pressures.
5. State the relationship between the Celsius and the thermodynamic scale of temperature.
6. What is (a) an isothermal change, and (b) an isobaric change?
7. Define an ideal gas.
8. State the characteristic gas equation.
9. What is meant by STP?
Practise Exercise 136 Multiple-choice questions on ideal gas laws
(Answers on page 298)
1. Which of the following statements is false?
(a) At constant temperature, Charles’ law applies.
(b) The pressure of a given mass of gas decreases as the volume is increased at constant temperature.
(c) Isobaric changes are those which occur at constant pressure.
(d) Boyle’s law applies at constant temperature.
2. A gas occupies a volume of 4 m3 at a pressure of 400 kPa. At constant temperature, the pressure is increased to 500 kPa. The new volume occupied by the gas is:
(a) 5 m3
(b) 0.3 m3
(c) 0.2 m3
(d) 3.2 m3
3. A gas at a temperature of 27°C occupies a volume of 5 m3. The volume of the same mass of gas at the same pressure but at a temperature of 57°C is:
(a) 10.56 m3
(b) 5.50 m3
(c) 4.55 m3
(d) 2.37 m3
4. Which of the following statements is false?
(a) An ideal gas is one that completely obeys the gas laws.
(b) Isothermal changes are those that occur at constant volume.
(c) The volume of a gas increases when the temperature increases at constant pressure.
(d) Changes that occur at constant pressure are called isobaric changes.
A gas has a volume of 0.4 m3 when its pressure is 250 kPa and its temperature is 400 K. Use this data in Questions 5 and 6.
5. The temperature when the pressure is increased to 400 kPa and the volume is in- creased to 0.8 m3 is:
(a) 400 K
(b) 80 K
(c) 1280 K
(d) 320 K
6. The pressure when the temperature is raised to 600 K and the volume is reduced to 0.2 m3 is:
(a) 187.5 kPa
(b) 250 kPa
(c) 333.3 kPa
(d) 750 kPa
7. A gas has a volume of 3 m3 at a temperature of 546 K and a pressure of 101.325 kPa. The volume it occupies at STP is:
(a) 3 m3
(b) 1.5 m3
(c) 6 m3
8. Which of the following statements is false?
(a) A characteristic gas constant has units of J/(kg K).
(b) STP conditions are 273 K and 101.325 kPa.
(c) All gases are ideal gases.
(d) An ideal gas is one that obeys the gas laws.
A mass of 5 kg of air is pumped into a con- tainer of volume 2.87 m3. The characteristic gas constant for air is 287 J/(kg K). Use this data in Questions 9 and 10.
9. The pressure when the temperature is 27°C is:
(a) 1.6 kPa
(b) 6 kPa
(c) 150 kPa
(d) 15 kPa
10. The temperature when the pressure is 200 kPa is:
(a) 400°C
(b) 127°C
(c) 127 K
(d) 283 K
Related posts:
Incoming search terms:
- characteristic gas equation
- characteristics gas equation
- characteristics gas formula
- an ideal gas is compressed at 130 °c isothermally butnotreversibly from 150 kpa absolute to 500 kpa absolute
- A gas is contained in a cylinder fitted with a piston The initial pressure of the gas is 1 3 bar and the initial volume is 0 03 m3 The gas is now heated isothermally until the volume of the gas increases to 0 1 m3 the work done by the gas is *
- a gas initially at 12 bar 216C and volume 9900cm is expanded in a cylinder
- a gas has a volume of 40 mL at a pressure of 800kPa if the pressure decreases to 200 kPa what is the fina volume of gas
- an ideal gas occupies o 5 m3 aat an absolute temperature of 600 kpa
- what is characteristic gas equation
- to what pressure does one fill 0 15 m^3 vessel at 25c
- A sealed container of volume 2 35 m3 contains 5 056g of oxygen gas If the temperature of the gas is 25 00 oC determine the absolute pressure of the gas Give your answer in Pa
- A certain gas is present in a 14 0 LL cylinder at 3 0 atmatm pressure If the pressure is increased to 6 0 atmatm the volume of the gas decreases to 7 0 LL Find the two constants kiki the initial value of kk and kfkf the final value of kk to verify whether
- characteristics of gas equation
- A gas has a volume of 350cm3 at 99kpa what will its volume be at 120kpa if the temperature remain constant
- What will be the volume of air at 327 °C if its volume at 27 °C is 1 5 m3 ?
- what is the formula for calculating the characteristic gas constant
- an increase in pressure of 100 kpa causes a certain volume of water to decrease by 0 005 percentage of its original volume CALCULATE BULK MODULUS
- at constant volume and 27 degree Celsius the pressure of gas is 2 atmosphere at 327 degree Celsius the pressure of gas in atmosphere is?
- Air in a 0 3-m cylinder is initially at a pressure of 10 bar and a temperature of 330K The cylinder is to be emptied by opening avalve and letting the pressure drop to that of the atmosphere
- An evacuated chamber has gas at a low pressure of 1 33×10−4 N/m2 and a temperature of 295 K
- An ideal gas occupies 0 4 m3 at an absolute pressure of 500 kPa What is the absolute pressure if the volume changes to 0 9 m3 and the temperature remains constant?
- an ideal gas is contained in a cylinder fitted with a piston initially the temperature is
- An ideal gas is contained in a cylinder of fixed length and diameter A piston does 30 J of work to compresses the gas and the internal energy of the gas increases by 10 J Which of the following statements is correct? 40 J of heat entered the gas 20 J of h
- at a pressure of 3atm air is pumped into the tubes of a cycle rickshaw
- characteristic equation of gas
- charachterstic equation of gas
- at a pressure of 3atm air is pumped into the tubes of a cycle
- at a pressure of 3 atm air is pumped into the tubes of cycle the volume of each tube is 0004m3
- calculate the volume of a given mass of gas at S t p if its volume in con at 27degree centigrade and under pressure of 700mm
- an ideal gas of mass 3kg is expanded from 7 bar pressure and 1 5 metric cube volume to 1 4 bar pressure and 4 5 metric cune volume change in internal energy is 525kj cv=1 047 kj/kg/k find gas constant change in enthalphy initial and final temp
- calculate the pressure of helium when the connecting valve is opened at constant temperature 27 c
- A gas originally at a pressure of Pi = 150 kPa and a volume of Vi = 3 0 m3 undergoes a cycle where it first is reduced to half its pressure at constant volume then expanded to triple its volume at constant pressure Then its pressure is doubled back to the
- A sealed container at 25oC contains a gas at a pressure of 104 kPa What is the pressure of the gas when it is heated to 225oC?
- 1m3 of air initialy at 110kn/m2 and 150oc is compressed according to the law PV1 3 = C in a cylinder to a final pressure of 1 4mn/m2 take R for air = 287j/kgk and CP = 1005j/kgk find (a) the volume and temperature of the air at the end of compression (b)
- 2 kg of a fluid at a pressure of 3 bar and a specific volume of 0 18 m3/kg is contained in a cylinder behind a piston The fluid then expands reversibly to a pressure of 0 6 bar The gas pressure and specific volume are related via the equation:
- 5kg of oxygen occupies a volume of 10
- a 2m^3 hydrogen at 30°C and 180 kpa abs
- A 3 27 m3 tank contains 100 kg of nitrogen at 175 K Determine the pressure in the tank using (a) the ideal-gas equation (b) the van der Waals equation and (c) the BeattieBridgeman equation
- a certain gas occupies a volume of 0 4m3 at a pressure of 100kPa and a temperature of 20
- a container with a volume of 1 8 m3 contains butane at a pressure of 7 6 mpa and atemperature of 280°c
- A cylinder contain 3 kg of air at pressure of 300 bar and temperature of 27°c Find the volume of air occupied by gas Assume R of air 287 j/kg°k
- You have 0 5 L of air at a pressure of 203 kPa and −70°C in a rigid sealed container What is the absolute temperature of the air?
- a cylinder of helium has a volume of 600cm the cylinder contains 200g of helium at temprature of 25C determine the pressure of the helium if the characteristic gas constant for helium is 2080 J(kgK)
- a gas has a density of 0 09kg/m^3 at a temperature of 0c and a pressure of 1 013 bar determine the characteristic gas constant
- A gas in a cylinder with a volume of 30 L and a pressure of 500 kPa is compressed by a piston to a volume of 10 L If the cylinder can only withstand a pressure of 1450 kPa will the cylinder be able to contain the gas or will it explode? answer key
- a gas occupies 150cm3 at 57c
- 287 j/kg*k
- a mass of 5kg of air is pumped into a container of volume 2 87m3 The characteristic gas constant for air is 287J/kgK The pressure when the temperature is 27°C is?
- a quantity of gas occupies a volume of 0 3
- a quantity of gas occupies volume of 0 4 at pressure of 100