There is still considerable disagreement about the exact nature of heat, but most authorities agree that it is a particular form of energy. Specifically, heat is a form of energy not associated with matter and in transit between its source and destination point. Furthermore, heat energy exists as such only between these two points. In other words, it exists as heat energy only while flowing between the source and destination.
So far this description of heat energy has been practically identical to that of work energy, the other form of energy in transit not associated with matter. The distinguishing difference between the two is that heat energy is energy in transit as a result of temperature differences between its source and destination point, whereas work energy in transit is due to other, nontemperature factors.
British Thermal Unit
Heat energy is measured by the British thermal unit (Btu). Each thermal unit is regarded as equivalent to one unit of heat (heat energy).
Since 1929, British thermal units have been defined on the basis of 1 Btu being equal to 251.996 IT (International Steam Table) calories, or 778.26 foot-pounds of mechanical energy units (work). Taking into consideration that one IT calorie equals 1⁄860 of a watt-hour, 1 Btu is then equivalent to about 1⁄3 watt- hour.
Prior to its 1929 redefinition, a Btu was defined as the amount of heat necessary to raise the temperature of one pound of water by one degree Fahrenheit. Because of the difficulty in determining the exact value of a Btu, it was later redefined in terms of the more fundamental physical unit.
Relationship Between Heat and Work
Energy is the ability to do work or move against a resistance. Conversely, work is the overcoming of resistance through a certain distance by the expenditure of energy.
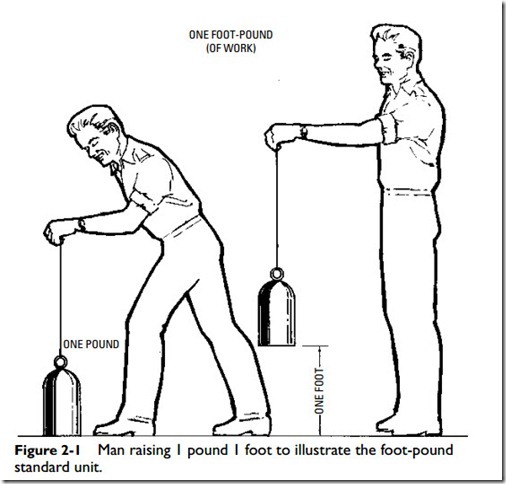
Work is measured by a standard unit called the foot-pound, which may be defined as the amount of work done in raising one
pound the distance of one foot, or in overcoming a pressure of one pound through a distance of one foot (Figure 2-1).
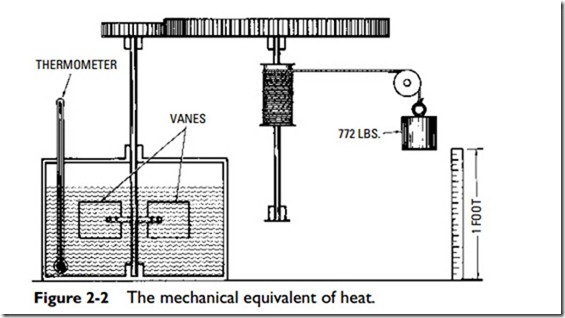
The relationship between work and heat is referred to as the mechanical equivalent of heat; one unit of heat is equal to ft-lb. This relationship (i.e., the mechanical equivalent of heat) was first established by experiments conducted in the nineteenth century. In 1843 Dr. James Prescott Joule (1818–1889) of Manchester, England, determined by numerous experiments that when 772 ft-lb of energy had been expended on 1 lb of water, the temperature of water had risen 1oF and the relationship between heat and mechanical work was found (Figure 2-2). The value 772 ft-lb is known as Joule’s equivalent. More recent experiments give higher figures and the value 778 (1 Btu = 778.26 ft-lb). (See the preceding section.)
Heat Transfer
When bodies of unequal temperatures are placed near each other, heat leaves the hotter body and is absorbed by the colder one until the temperatures are equal to each other. The rate by which the heat is absorbed by the colder body is proportional to the difference of temperature between the two bodies—the greater the difference in temperature, the greater the rate of flow of the heat.
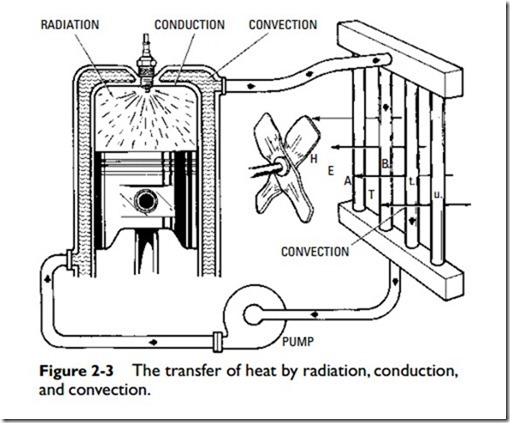
Heat is transferred from one body to another at lower temperature by any one of the following means (Figure 2-3):
1. Radiation
2. Conduction
3. Convection
Radiation, insofar as heat loss is concerned, refers to the throw- ing out of heat in rays. The heat rays proceed in straight lines, and the intensity of the heat radiated from any one source becomes less as the distance from the source increases.
The amount of heat loss from a body within a room or building through radiation depends upon the temperature of the floor, ceiling, and walls. The colder these surfaces are, the faster and greater will be the heat loss from a human body standing within the enclosure. If the wall, ceiling, and floor surfaces are warmer than the human body within the enclosure they form, heat will be radiated
from these surfaces to the body. In these situations a person may complain that the room is too hot.
Knowledge of the mean radiant temperature of the surfaces of an enclosure is important when dealing with heat loss by radiation. The mean radiant temperature (MRT) is the weighted average temperature of the floor, ceiling, and walls. The significance of the mean radiant temperature is determined when compared with the clothed body of an adult (80°F, or 26.7°C). If the MRT is below 80°F, the human body will lose heat by radiation to the surfaces of the enclosure. If the MRT is higher than 80°F, the opposite effect will occur.
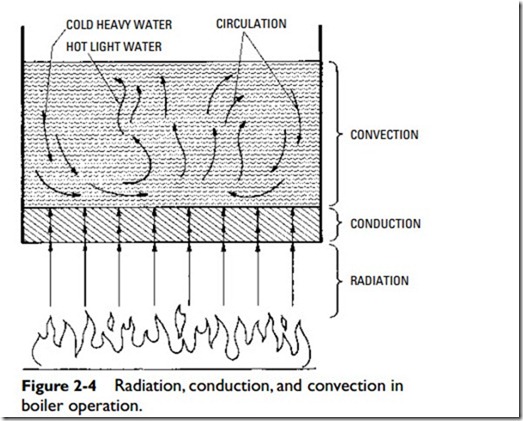
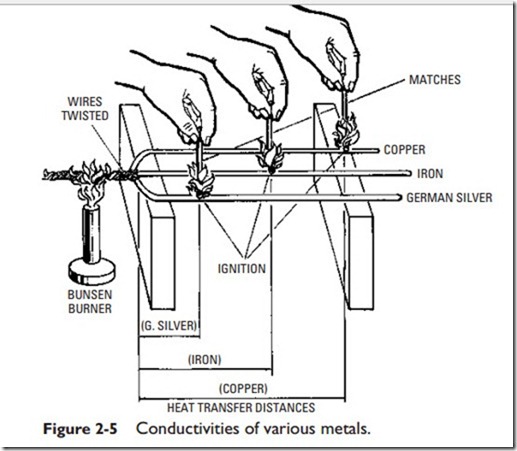
Conduction is the transfer of heat through substances, for instance, from a boiler plate to another substance in contact with it (Figure 2-4). Conductivity may be defined as the relative value of a material, compared with a standard, in affording a passage through itself or over its surface for heat. A poor conductor is usually referred to as a nonconductor or insulator. Copper is an example of a good conductor. Figure 2-5 illustrates the comparative heat conductivity rates of three frequently used metals. The various materials used to insulate buildings are poor conductors. It should be
pointed out that any substance that is a good conductor of electricity is also a good conductor of heat.
Convection is the transfer of heat by the motion of the heated matter itself. Because motion is a required aspect of the definition of convection, it can take place only in liquids and gases.
Figure 2-4 illustrates how radiation, conduction, and convection are often interrelated. Heat from the burning fuel passes to the metal of the heating surface by radiation, passes through the metal by conduction, and is transferred to the water by convection (i.e., circulation). Circulation is caused by a variation in the weight of the water due to temperature differences. That is, the water next to the heating surface receives heat, expands (becomes lighter), and immediately rises as a result of displacement by the colder and heavier water above.
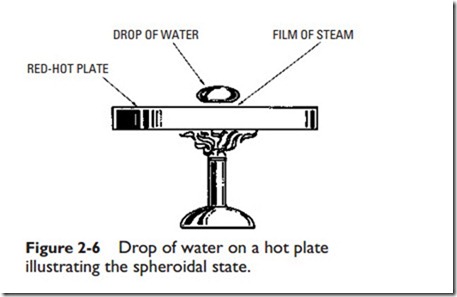
Proper circulation is very important, because its absence will cause a liquid, such as water, to reach the spheroidal state. This, in turn, causes the metal of the boiler to become dangerously overheated. A liquid that has reached the spheroidal state is easy to recognize by its appearance. When liquid is dropped upon the surface of a highly heated metal, it rolls about in spheroidal drops