FUEL OILS
Fuel oils are hydrocarbon mixtures obtained from crude petro leum by refining processes. They may be divided into the follow ing six classes or grades:
1. No. 1 fuel oil for vaporizing pot-type and other burners designed for this type fuel.
2. No. 2 fuel oil for general purpose domestic heating not requiring a lighter No. 1 fuel oil.
3. No. 3 fuel oil (obsolete since 1948).
2. No. 4 fuel oil for installations not equipped for preheating.
3. No. 5 fuel oil for installations equipped for preheating.
4. No. 6 fuel oil for burner installations equipped for preheat ing with a high viscosity fuel.
This classification of fuel oils is based on a number of char acteristics, including: (1) viscosity; (2) flash point; (3) pour point; (4) ash content; ( 5) carbon residue, and ( 6) water and sediment content.
All of ·these characteristics are important to consider when selecting a fuel oil. For example, the water content will deter mine its suitability for outdoor torage. A low viscosity indicates a fuel oil with good flow characteristics.
A No. 1 fuel oil has the lowest viscosity ( 1.4 – 2.2). This is a dis·tillate oil used in atomizing and similar type burners, because it can be easily broken down into small droplets.
A No. 2 fuel oil is a distillate oil used in domestic oil burners that do not require preheating. It is slightly heavier than a No. 1 fuel oil. No. 4 fuel oil is slightly heavier than No. 2, and will work in some oil burners without preheating.
No. 2 and No. 4 fuel oils contain the highest ash content (.10 •to .50% by weight respectively). The other grades contain little or no ash. Although slagging (i.e. the formation of noncom bustible deposits) is commonly associated with solid fuels such as coal, this problem also occurs in oil burning equipment (see ASH, SLAG, AND CLINKER FORMATION in this chapter).
No. 5 and No. 6 (or Bunker C) fuel oils are heavier ·than the other grades and require preheating. In automatically operated oil burners, the preheating of the fuel oil must be completed be fore it is delivered for combustion. When the fuel oil has reached a suitable atomizing temperature, the preheating is considered completed.
PS 300 and PS 400 are heating oils used on the West Coast. and roughly correspond to No. 5 and No. 6 fuel oils respectively. PS 300 is the lighter of the •two, and is used primarily for domes tic heating purposes. PS 400 is heavier, requires preheating, and is used for industrial purposes.
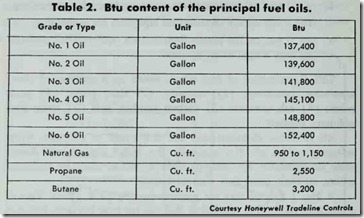
The grade of fuel oil to be used in an oil burner is specified by the manufacturer. This should also be stipulated on the label of the Underwriters’ Laboratories, Inc., and Underwriters’ Lab oratories of Canada. Table 2 compares the Btu content of the principal fuel oils.
COAL
Coal is a solid fuel created from deposits of ancient vegeta tion that have undergone a series of metamorphic changes result ing from pressure, heat, submersion, and other natural formative processes occurring over a long period of time. Because of the different combinations of these processes acting on the vegetation and the widely differing forms of vegetation involved, coal is a complex and nonuniform substance.
The formation of peat represents a pre-coal stage of development. After increased pressure and time, lignite begins to form. The next stage is semibituminous, followed by bituminous, and then anthracite. Each of these stages is characterized by an in crease in the hardness of the coal. Anthracite is the hardest of the coals. Graphite represents a post-coal developmental stage, and cannot be used for heating purposes.
Coal is often classified according to the degree of metamorphic change to which it has been subjected into the following categories:
1. Anthracite,
1. Bituminous,
2. Semibituminous,
4. Lignite.
Anthracite is a clean, hard coal that burns with little or no luminous flame or smoke. It is difficult to ignite, but burns with a uniform, low flame once the fire is star,ted. Anthracite contains approximately 14,440 Btu’s per pound, and is used for both do mestic and industrial heating purposes. Its major disadvantage as a heating fuel is its cost.
Anthracite coal is divided by size into a number of different
grades. Each of these grades (e.g. egg size, buckwheat size, pea size) is suitable for a specific size fire-pot. They are described in greater detail in Chapter 3, Volume 2 (COAL FIRING METHODS).
A great amount of smoke will result if it is improperly fired. The term “bituminous coal” actually covers a whole range of coals many of which have widely differing combustion characteristics. Some of the coals belonging to this classification are hard, whereas others are soft.
The available heat for bituminous coal ranges from a low of 11,000 Btu’s per pound (Indiana bituminous) to a high of 14,100 Btu’s per pound (Pocahontas bituminous). The heat value of the latter approximates that of anthracite (about 14,400 Btu’s per pound); however, unlike anthracite coal, it is available in far greater supply, a factor that makes it a very economical solid fuel to use.
Semibituminous coal is a soft coal that ignites slowly and burns with a medium length flame. Because it provides so little smoke, it is sometimes referred to as smokeless coal.
Lignite (sometimes referred to as brown coal) ignites slowly,
produces very little smoke, and contains a high degree of mois ture. In structure, it is midway between peat and bituminous coal. Lignite contains approximately half the available heat of anthra cite (or about 7400 Btu’s per pound), and burns with a long flame. Its fire is almost smokeless, and it does not coke, a characteristic which it shares with anthracite.
Lignite is considered a low-grade fuel, and its calorific value is low when compared with .the other coals. Moreover, it is difficult to handle and store.
COKE
Coke is the infusible, solid residue remaining after the distilla tion of certain bituminous coals, or as a by-product of petroleum distillation. Coke may also be obtained from petroleum residue, pitch. and other materials representing the residue of destructive distillation.
Coke will ignite more quickly than anthracite, but less readily than bituminous. It burns rapidly with little draft. As a result, all openings or leaks into the ash pit must be closed tightly when the coke is being burned.
Since less coke is burned per hour per square foot of grate than coal, a larger grate is required and a deep fire-pot is neces sary to accomodate the thick bed of coal. Since coke contains very little hydrogen, the quick flaming combustion which char acterizes coal is not produced, but the fire is nearer even and regular.
The best size of coke recommended for general use, for small fire-pots where the full depth is not over 20 inches is that which passes over a one inch screen and through a one and a half inch screen. For large fire-pots where ,the fuel can be fired over 20 inches deep, coke which passes over a one inch screen and through a three inch screen can be used, but a coke of uniform size is always more satisfactory.
BRIQUETTES
Briquettes are a solid fuel prepared from coal dust and fines (i.e. finely crushed or powdered coal) by adding a binder and holding it under pressure.
The binder is commonly pitch or coal tar, but other substances and materials have also been used. A briquette made with a pi,tch binder has the highest calorific value, exceeding that of any coal. The ash content is generally less than that of coal. Briquettes have been made from lignite coal dust and fines without a binder (only pressure being used).
COAL OIL
Coal oil is a heating fuel formerly derived from coal tar. For merly it enjoyed widespread use, but i’t has now been largely
replaced by petroleum based products. It is also called paraffin oil or kerosene. Today, coal oil (kerosene) is obtained from petro leum through a refining process.
WOOD AS A FUEL
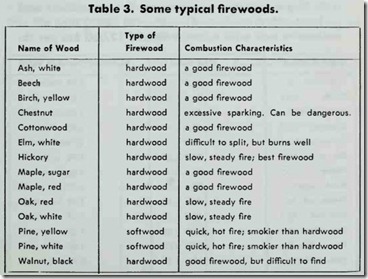
A good, well-seasoned hardwood will provide approximately half as much heat value per pound as does good coal (Table 3). Wood is easy to ignite, burns with little smoke, and leaves com paratively li,ttle ash. On the other hand, it requires a larger storage space (commonly outdoors) and more labor is involved in its preparation.
Wood is commonly sold by the cord for heating purposes. A standard cord measures 4 feet high by 8 feet wide and contains wood cut to 4 foot lengths for a total of 128 cubic feet. Only about 70% of a tandard cord actually represents the wood con tent, however, bec.ause of the existence of air spaces between the wood.
The heat value of wood depends upon the type of wood being burned. Heat values per cord for a number of different types of
wood are given in Tables 4 and 5. The data used to compile the tables was obtained from “Use of \Vood for Fuel” (U.S. Depart ment of Agriculture Bulletin No. 753). The wood heat values are determined for cords containing approximately 90 cubic feet of solid wood (i.e. about 70 % of a standard cord). Note that the heat values for both green and “dry” wood ( 12% moisture con tent) are given in Tables 4 and 5. It is important to remember that the amount of moisture in a wood will effect its burning characteristics. A green wood will burn much more slowly than a drier one, and its fire will be more difficult to start.
All woods can be divided into either hardwood or softwood types. Contrary to popular belief, the basic difference between the two groups is not the hardness of the wood. In other words, a wood that can be classified as a softwood is not necessarily softer than a hardwood. These terms do not refer to the physical properties of the wood but rather to its classification as a coni ferous (needle or cone bearing) or deciduous (broad-leafed) tree. A softwood, for example, comes from a coniferous ·tree, whereas a hardwood is obtained from a deciduous tree. You will find that some soft wood trees produce wood that is harder than the wood obtained from some hardwood trees.
Chimney or flue fires are always a hazard when using wood as a heating fuel. Resins and soot collect in these areas over a period of time, and can be ignited if there is a ftareup of the fire. The possibility of this happening can be eliminated or greatly reduced by cleaning the chimney or flue from time to time.
Softwoods (e.g. white pine, yellow pine, etc.) contain greater amounts of resin than do the hardwoods. This tends to give them a higher calorific value ,than the hardwoods and increased in flammability, but it also results in more smoke.
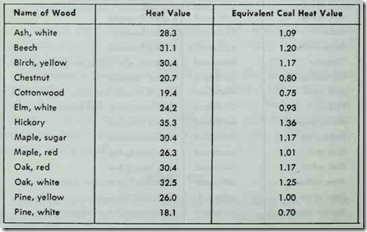
Table 4. Heat value per cord (million Btu’s) of wood with 12 percent moisture content. The equivalent coal heat values are based on one ton (2000 lbs.) of anthracite coal with a heat value of 13,000 Btu per lb.
ASH, SLAG, AND CLINKER FORMATION
Ash is the noncombustible mineral residue that remains in the furnace or boiler after the fuel has been thoroughly burned. During the combustion process, the combustible portion of the fuel is consumed and the noncombustible ash remains in place. As the process continues, ,the ash residue is subjected ,to a certain degree of shrinkage. This shrinkage results in certain portions of the ash fusing together and forming more or less fluid globules or slag. This process is referred to as slagging.
Under the proper temperature conditions, the fluid slag glob ules can solidify and form clinkers in •the fuel bed or deposits on the heating surfaces of the furnace or boiler. Clinkers can obstruct the necessary air flow to the fire, and result in its reduced effi ciency or extinction. These deposits on the heating surfaces will reduce their heating capacity, and must be removed. Suggestions for removing clinkers are given in Chapter 3, Volume 2 (COAL FIRING METHODS).
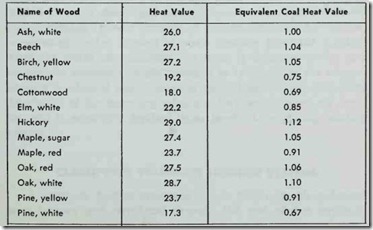
Table 5. Heat value per cord (million Btu’s) of green wood. The equivalent coal heat values are based on
one ton (2000 lbs.) of anthracite coal with a heat value of 13,000 Btu per lb
SOOT
Soot is a fine powder consisting primarily of carbon produced by the combustion process. Because of its extreme light weight, it frequently rises with the smoke from the fire and coats the interior walls of the chimney and flue. Although ·the heat loss from the insulating effect of a soot layer is small (generally under 6% ) , it can cause a considerable rise in the stack temperature. Soot accumulation can also clog the flues, thereby reducing the draft and resulting in improper combustion. Soot may be blasted loose from .the walls of the chimney or flue with a jet of com pressed air, or sucked out with a vacuum cleaner. Another method is to use a brush to remove the accumulated soot layer from the walls.