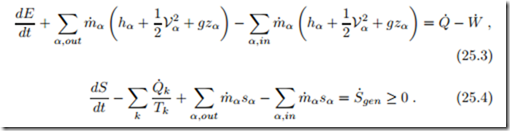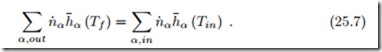First and Second Law for Combustion Systems
The goal of combustion is to produce heat, either for heating purposes or for conversion into work in heat engines. Since the changes in chemical energy are reflected in the proper values for enthalpies (23.20) and entropies (23.22), the first and second law for a combustion system have the usual form (9.9, 9.10),
We shall consider mainly open systems, e.g., combustion chambers, at steady state and ignore kinetic and potential energies, so that, now written with mole flows instead of mass flows, and with molar enthalpy and entropy, the first and second law read
The mole flows n˙ α must be determined by analysis of the combustion process as shown in the previous sections.
Adiabatic Flame Temperature
If the combustion system is adiabatic, and no power is exchanged, the first law for isobaric combustion reduces to
The temperature Tf of the combustion product is the adiabatic flame temperature.
Example: Adiabatic Flame Temperature
As an example, we compute the adiabatic flame temperature for the combustion process studied in Section 25.3, when the incoming fuel and airstreams are at reference temperature and pressure, Tin = T0, p = p0. Then, the enthalpies for the incoming streams are
Here we used that the products are ideal gases, so that the enthalpies depend only on temperature. From the tables we find the values