Compression and Cooling
The objective of the use of compression in air-conditioning is to cool the air being conditioned. It is important to note, however, that it is not the air that is compressed, but the refrigerant gas used in the coils of the air-conditioning unit. The low temperature is produced by the expansion and contraction of the refrigerant gas. When a gas is compressed, both its pressure and temperature are changed in accordance with Boyle’s and Charles’s laws.
The English scientist Robert Boyle (1627–1691) determined that the absolute pressure of a gas at constant temperature varies inversely as its volume. Somewhat later, the French scientist Jacques Charles (1745–1823) established that the volume of a gas is proportional to its absolute temperature when the volume is kept at constant pressure. These findings came to be known as Boyle’s and Charles’s laws respectively. In Tables 8-3 and 8-4 a series of relations based on these two laws are tabulated for convenient reference.
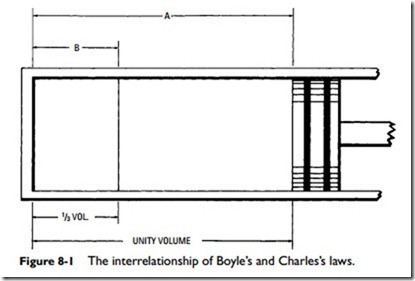
A more simplified explanation of the interrelationship of these two laws may be gained with the aid of the cylinder illustrated in
Figure 8-1. If the cylinder is filled with air at atmospheric pressure (14.7 psi) represented by volume A, and piston B is moved to reduce the volume by, say, 1⁄3 of A, as represented by B, then according to Boyle’s law, the pressure will be tripled (14.7 X 3 =
lbs absolute or 44.1 14.7 = 29.4 gauge pressure). According to Charles’s law, a pressure gauge on a cylinder would at this point indicate a higher pressure than 29.4 gauge pressure because of the increase in temperature produced by compressing the air. This is called adiabatic compression if no heat is lost or received externally.