Electrolyzers
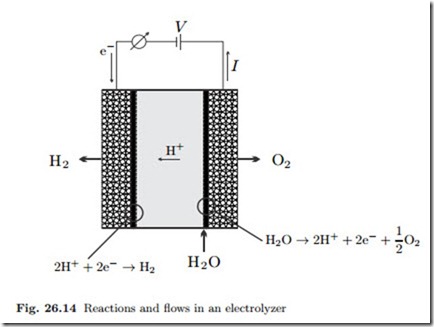
In fuel cells, hydrogen and oxygen combine to produce electrical energy and water. In electrolyzers, the opposite takes place: electrical energy is used to split water into hydrogen and oxygen. Figure 26.14 shows the basic reactions taking place, and indicates the flows of hydrogen, oxygen, water, protons, and electrons.
The power consumption of the electrolyzer is W˙ E = −VE I and the amount of hydrogen produced is equal to the reaction rate Λ = I/2F . Thus, the work required for the production per moler of hydrogen, wH2 , is directly proportional to the electrolyzer potential,
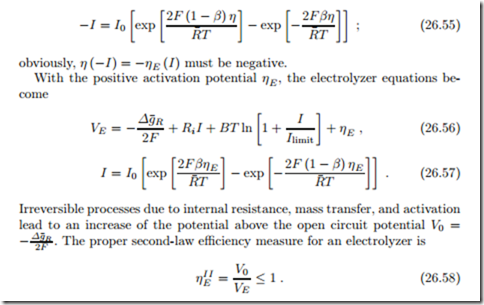
Since all flows are just in opposite direction as in a fuel cell, the potential and power consumption for an electrolyzer follow from the equations for fuel cells simply by inverting the sign of the electrical current. Then, from (26.49), the electrolyzer potential becomes
where η (−I) solves the Butler-Volmer equation for negative current,
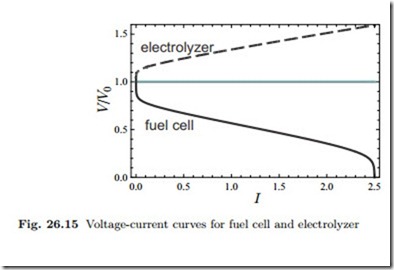
Figure 26.15 compares the voltage-current curves for fuel cell and electrolyzer to the open circuit potential. The gap between the curves is the loss to irreversible processes.