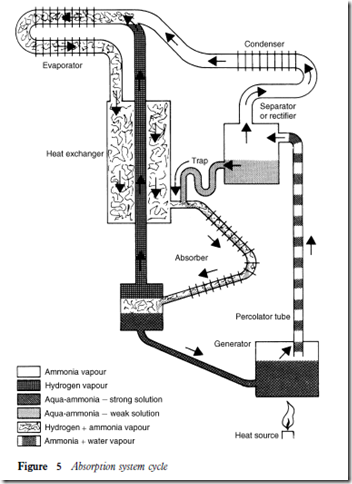
The domestic absorption system
The modern domestic absorption refrigerator or ‘gas refrigerator’ operates on the principle of using heat to produce cold. It employs a mixture of hydrogen and ammonia with water (aqua-ammonia) as the cooling agent: water has an affinity for ammonia, and the hydrogen speeds up the process of evaporation.
The heat source may be town gas or, in the case of smaller systems designed for use in caravans, camp sites etc., propane, butane or even paraffin. An all-electric version has a small heater element to provide heating.
The valve assemblies which regulate the gas flow incorporate a small pilot jet to ignite the gas, as the valve is energized electrically. The evaporating temperature is controlled by a thermostat which in turn energizes the gas valve or heater element, depending upon the setting of the thermostat (cold control).
Operation
When the thermostat energizes the valve or heater, heat is applied to the ammonia and water mixture in the generator. The liquid then boils off the pass through a small tube (percolator tube), in much the same way as coffee in a percolator, to enter the separator or rectifier. From there it circulates by gravity.
The ammonia vaporizes faster than the water and the aqua-ammonia separate in the rectifier; the ammonia vapour rises to the condenser and the water drains back to the absorber to be recirculated. The lighter ammonia vapour rising from the condenser coils changes back to a liquid to drain into the evaporator, where it mixes with the hydrogen vapour from the absorber.
Heat from the stored product in the food compartment of the refriger- ator will vaporize the ammonia liquid in the evaporator. The mixture of ammonia vapour and hydrogen, being heavier than either of the two gases alone, passes to the absorber where it meets the water coming from the recti- fier. The ammonia is then absorbed by the water and, when the water has absorbed as much vapour as it can hold, the vapour returns to the evaporator.
At maximum working conditions (maximum heat at generator) the pressure on the high side of the system (condenser and absorber) will be approximately 14 bar with a relative temperature of 36 °C. Assuming that the refrigerator is in an average ambient temperature of 21 °C, the heat rejection will be quite reasonable with a 15 ° temperature (36 21 °C). Pressure on the low side of the system will be 12.6 bar hydrogen and 1.2 bar ammonia, so the relative temper- ature of the evaporator will be -15 °C. At this temperature, heat exchange from the food products to the evaporator will be at an average 3 °C, producing a 12 ° temperature difference [3 to – 15 D 12 °].
ervicing
Since this is a completely sealed system containing two gases, very little can be achieved by the service engineer except the replacement of a thermostat, valve assembly or heater element.
The pilot jet and valve should be kept clean and the refrigerator should be carefully levelled at the time of installation and checked when service is carried out.
Figure 65 shows the absorption system cycle.