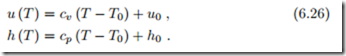Monatomic Gases (Noble Gases)
For monatomic gases, i.e., the noble gases helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn), the specific heats are true constants with the values
and the caloric equation of state follows from straightforward integration as
With cp = const, the integration in (6.20) can be performed easily, and the entropy becomes
Since the resulting expressions for the thermodynamic quantities of monatomic gases are rather simple, these are typically not tabulated.
Related posts:
Boiler and Furnace Conversion:Oil Tanks and Oil Piping
Radial-Flow Gas Turbines:The scroll and stator blades.
Coal Furnaces,Wood Furnaces, and Multi-Fuel Furnaces:System Accessory Devices
Air Cleaners and Filters:Performance Lights
Steam Heating Systems:Steam Boilers
Energy Conservation Measures:Energy Considerations for Buildings
Problems on two-dimensional cascades:
Service diagnosis and repairs:Compressor motor burn-out: system flushing
Ventilation and Indoor Air Quality:Indoor Air Quality Effects on Health and Comfort