Conventional fuel
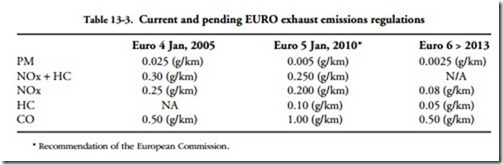
Serious efforts are underway to ameliorate some of the worst effects associated with conventional fuels. In accordance with the Kyoto Protocols, the EU has agreed to reduce emissions of CO2, which are believed to be the primary cause of global warming. Because diesels produce less carbon dioxide than SI engines, European governments promote their use with tax breaks and diesel-friendly emissions regulations (Table 13-3). Many experts believe that, by 2008, half of the new cars registered in Western Europe will be diesel powered.
Some translation is needed:
• PM (particulate matter) refers to the solids in diesel exhaust that are some- times visible as soot.
• NOx (nitrogen oxides) is a blanket term for various oxides of nitrogen, such as NO and NO2.
• HC (hydrocarbons) come about as the result of incomplete combustion of
fuel and lube oil.
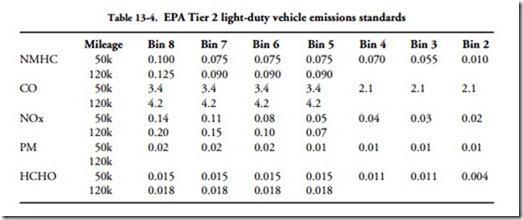
• CO (carbon monoxide) is an odorless gas, lethal at high concentrations. Table 13-4 lists the U.S. Environmental Protection Agency (EPA) 50-state standards for light vehicles that were phased in during 2004 with full compliance scheduled for 2010. These standards apply both to diesel- and gasoline-fueled vehicles. Heavy pickup trucks, such as the Silverado, and large SUVs are partially grandfa- thered until 2008, when they will have to meet the same standards as other vehicles.
What the EPA calls NMHC (nonmethane hydrocarbons) is known as HC. HCHO, better known as formaldehyde, is a powerful carcinogen.
In the EPA scheme of things, manufacturers are free to classify their engines into any of the seven bins shown in the table. But the fleet average, that is, emissions from all engines the manufacturer sells, must conform to Bin 5 standards after 2010. Rather than juggle the mix by counter-balancing relatively dirty Bin 7 or 8 engines with super-clean Bin 2 or 3 models, most manufacturers aim at producing Bin 5 engines across the board.
NOx and PM
Bin 5 limits NOx to 0.07 g/mile and PM to 0.01 g/mile, which are, respectively, one-sixth and one-half of EURO 4 limits. The PM standard approaches that of rubber dust from vehicle tires. Some explanation is required to understand why the EPA has taken these draconian measures, which affect diesel engines far more than their SI counterparts.
Both NOx and PM pose serious health risks, especially for urban populations. NOx reacts with sunlight to produce smog and is the pollutant most responsible for low-level ozone. The health effects of long-term exposure to ozone include chronic respiratory problems, reduced potential for exercise, and more frequent emergency- room visits. Ironically some of the most severe ozone exposure occurs in suburbs, downwind of city traffic.
PM, sometimes visible as the sooty component of diesel exhaust and felt as a stinging sensation on the face, presents an array of health problems, one of which is lung cancer. According to the American Lung Association, 50,000 Americans die each year because of exposure to PM, taken for purposes of the study as particles of less than 10 j.Lm in diameter. No one has identified the biological mechanism involved, nor does the association try to distinguish between diesel PM, dust, and tobacco smoke. But there is a strong statistical correlation between inhaling PM and dying early. Perhaps a more telling statistic is that between 1980 and 2000, asthma increased 160% in American children under the age of four. African-American chil- dren, who live in disproportionate numbers in urban areas with high levels of diesel PM, die from asthma at the rate 11.5 per million, or more than four times the rate for white children.
Diesel engines are relatively clean in terms of HC and CO, but produce large amounts of NOx and PM. By insisting that all engines, regardless of fuel used, meet the same emissions standards, the EPA has moved to close what it identifies as the “health gap” between CI and SI.
Unfortunately, the two most toxic pollutants in diesel exhaust are the most dif- ficult to control. The same high combustion-chamber temperatures that burn off HC and convert CO to CO2 generate NOx and PM.
Designers were able to meet EPA Tier 1 and EURO 4 NOx and PM standards with in-cylinder controls, such as the use of exhaust gas recirculation and retarded injection to reduce flame temperatures. However, Tier 2 and pending European stan- dards cannot be met by engine modifications alone. For that, one needs exhaust aftertreatment.
NOx aftertreatment
The high level of air in diesel exhaust makes the three-way catalytic converters used on SI automobiles impractical for CI engines. But it is possible to engineer around the problem, although at the price of some complexity.
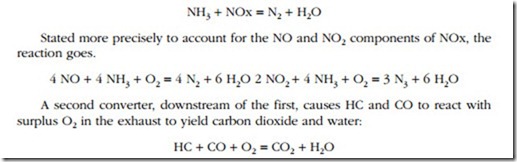
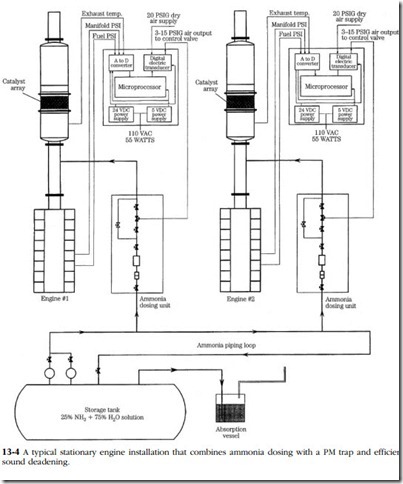
Figure 13-4 illustrates the system developed by Houston Industrial Silencing for standby generators and other stationary applications. It covers all bases with a highly efficient muffler, two catalytic converters and a PM trap. The microprocessor- controlled injection system delivers the correct amount of ammonia (NH3), to the first-stage converter. Ammonia reacts with NOx to produce free nitrogen and water.
A solution of 75% water and 25% ammonia is normally used as the oxidant. The dry (anhydrous) form of ammonia can also be injected directly into the exhaust or mixed with steam prior to injection. In any event, the rate of injection must be pre- cisely calibrated for each installation by sampling NOx concentrations at the exhaust outlet.
When using platinum as the catalyst, the system is said to reduce 90% or more of the NOx present and at least 95% of the CO. HC conversion rates are comparable to those for CO. Platinum operates in a temperature band of 460°–540°F, attainable by a well-engineered installation operating under constant load.
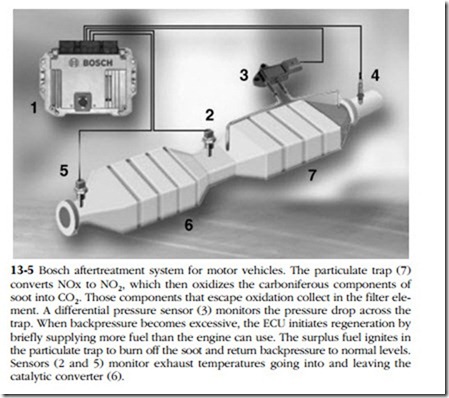
NOx-reduction systems for motor vehicles pose more formidable problems (Fig. 13–5). These systems must be compact, capable of operating under variable loads and, when used on heavy trucks, must function 290,000 miles without maintenance. For automobiles, the zero-maintenance requirement is 100,000 miles.
Ford and Bosch employ a solution of urea (NH2)2CO and water as the ammonia carrier. Urea breaks down into ammonia (NH3) in the catalytic converter, which reacts with NOx as described above to produce water and nitrogen gas. Under ideal temperature conditions, these systems are claimed to oxidize more than 90% of avail- able NOx. The urea component amounts to about 5% of diesel fuel consumption.
A delivery system filters the urea, atomizes it with compressed air, and adjusts delivery to engine load, speed and exhaust temperature. One or more oxygen sen- sors continuously sample the exhaust to provide feedback.
AdBlue, the Bosch marketing name for urea (actually a common fertilizer), is available at service stations in Germany. Ford and Mercedes-Benz have received EPA approval to introduce these converters in the U.S. although the agency would prefer a true zero-maintenance system.
Honda’s 2.2 L i-CTDi, scheduled for introduction before 2010 when Tier 2, Bin 5 comes into effect, does away with urea injection. The catalytic converter is coated with two absorbent layers. The outer layer traps NOx during lean-burn operation. At inter- vals, the engine goes rich. Hydrogen, obtained from the unburnt fuel in the exhaust, reacts with the captive NOx to produce ammonia (NH3). The second layer of
absorbent material stores NH3 until the engine returns to its normal, lean-burn operation. At this point, the ammonia reacts with NOx to yield water and nitrogen gas.
The system should have very little effect upon fuel consumption: according to a Honda spokesman, when running at 60 mph the engine operates lean for three minutes, then rich for five seconds. Amazing.
PM trap
The trap, also called a diesel particulate filter (DPF), mounts upstream of the cat- alytic converter. Because of the high temperatures involved, filter elements are made of porous ceramic or sintered metal. When the filter clogs, the computer senses the restriction as excessive backpressure, and orders injection late during the expansion stroke. Raw fuel floods the trap and spontaneously ignites, burning off carbon and soot accumulations. Periodically, the trap must be disassembled for removal of ash and other hard deposits.
Peugeot-Citroen employs a honeycomb silicon carbide PM trap that is cleaned with a combination of fuel and a proprietary additive, known as Eolys. Since Eolys is not the sort of stuff you buy at the corner gas station, the EPA will probably not approve it.
Ultra-low sulfur diesel
Sulfur clogs PM traps and poisons catalytic converters, and is in itself a source of PM. Consequently, the EPA ruled that the sulfur content of Nos.1 and 2 diesel be reduced from 500 ppm to 15 ppm. ULSD (ultra-low sulfur diesel) may be used in any vehicle engine, and is mandatory for 2007 and later models.
The changeover, which begun in mid-2006, put at least one small refinery out of business, but has generally progressed smoothly. One concern was that hydro- genation, the process refiners use to remove sulfur, also lowers the lubricity of ULSD. Additives bring the fuel up to 500 ppm lubricity levels. Aside from scattered reports, difficult to document, there seems to have been no serious problems in this regard.
Because diesel engines live so long, it will be decades before the full effect of Tier 2 standards and ultra-low sulfur fuel make themselves felt. If EPA projections are correct, our grandchildren should see a 2.6 million ton reduction in annual emis- sions of NOx and a 100,000 ton reduction in PM.
Meanwhile, the regulatory climate in the U.S. is changing. The reality of global warming has now been accepted by most opinion leaders, including many of the same conservatives who led the fight against ratification of the Kyoto Accords. Effects of other emissions are, for the most part, invisible and detected only by statistics on hospital admissions and longevity. Signs of catastrophic climate change are more difficult to ignore.
Environmentalists hailed the recent Supreme Court decision that empowers the EPA to treat carbon dioxide emissions under its general mandate to protect the envi- ronment. Other pollutants can be converted chemically into less noxious com- pounds. But no conversion technology, no magic bullet, exists for CO2. The only way to reduce carbon dioxide emissions is to burn less fuel. As the most efficient form of internal combustion, the diesel engine has a bright future.