NITROGEN OXIDE CAPTURE
As with sulfur dioxide, it is possible to remove nitrogen oxide after it has been formed in the flue gas of a power plant. The process involves the injection of either ammonia gas or urea into the flue-gas stream. Either chemical reacts with the nitrogen oxide present, converting the oxides into nitrogen and water.
If the ammonia or urea is injected into the hot flue-gas stream where the temperature is between 870 oC and 1200 oC, the reaction will occur spontaneously. This is called selective noncatalytic reduction (SNCR). At lower temperatures, the reaction is too slow, so a special metal catalyst is necessary to stimulate the reaction process. Where a catalyst is used, the process is called selective catalytic reduction (SCR).
SNCR will remove between 35% and 60% of the nitrogen oxide from the flue-gas stream. However, if care is not taken it can lead to contamination of fly ash with ammonia and to ammonia slip—the release of excess ammonia into the atmosphere. Nevertheless, it has been utilized at power plants in several parts of the world.
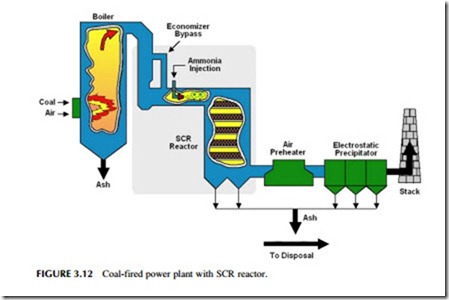
More widely used than SNCR is SCR. SCR units are commonly found on gas turbine power stations but may also be fitted to a coal-fired power plant where low nitrogen oxide combustion strategies do not reduce the emissions levels to below the regulatory limits (Figure 3.12). Typical flue-gas temperatures in an SCR unit are 340–380 oC. The hot gases pass over a solid catalyst that is normally made from a vanadium-titanium-based material or from a zeo- lite. The system is generally capable of removing 70–90% of the nitrogen oxide emissions from a flue-gas stream. Ammonia slip, although still possible, tends to be less of a problem.
There are two major drawbacks to SCR. First, it can only be used with low sulfur coals (up to 1.5% of sulfur), and second, it is expensive. The catalyst requires changing every three to five years. In addition, sulfur in coal entering the SCR can be converted into trioxide (SO3), which becomes highly corrosive on contact with water when it forms sulfuric acid. This has to be carefully con- trolled or it can be extremely damaging both in the power plant and in the atmosphere.
It is also possible to remove nitrogen oxide using a wet scrubbing system. The reagent sprayed into the flue-gas stream in this case is a solution sodium hydroxide, although hydrogen peroxide has also been used. The reaction that takes place leads to the formation of nitrites and nitrates that must be isolated for disposal. Wet scrubbing is not normally used in power stations for nitrogen oxide removal.