SULFUR DIOXIDE REMOVAL
There are no combustion strategies that can be used to control the generation of sulfur dioxide in PC plants. If sulfur is present in coal it will be converted into sulfur dioxide during combustion. The only recourse is to capture the sulfur, either before the coal is burned using a coal-cleaning process, or after combustion using some chemical reagent inside the power plant.
There are many chemicals that are potentially capable of capturing sulfur dioxide from the flue gases of a power station but the cheapest to use are lime and limestone. Both are calcium compounds that will react with sulfur dioxide to produce calcium sulfate. If the latter can be made in a pure-enough form it can be sold into the building industry for use in wall boards.
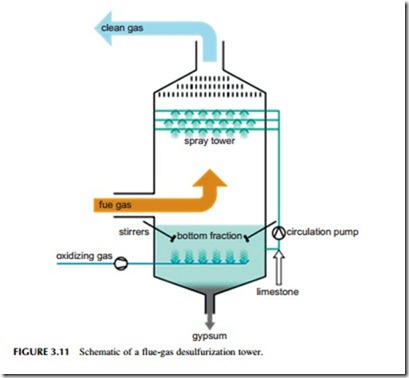
One of the simplest methods of capturing sulfur dioxide is to inject one of these sorbent materials into the flue-gas stream as it exists the furnace (Figure 3.11). Reaction then takes place in the hot gas stream and the resultant particles of calcium sulfate, and of excess sorbent, are captured in a filter down- stream of the injection point.
Depending on the point of injection of the sorbent, this method of sulfur removal can capture between 30% and 90% of the sulfur in the flue-gas stream.
The cheapest, and least effective, method (30–60% capture efficiency) is to inject the sorbent directly above the furnace. Injection later in the flue-gas stream is more expensive but can remove up to 90% of the sulfur.
Sorbent injection into a flue-gas stream is one of the cheapest methods of capturing sulfur but it is not the most efficient. The best established method of removing most of the sulfur from the flue gas of a power plant is with a flue-gas desulfurization (FGD) unit, also called a wet scrubber. The FGD unit comprises a specially constructed, tall chamber through which the flue gas passes. In a typical scrubber, a slurry of water containing 10% lime or limestone is sprayed into the path of the flue gas where it reacts, capturing the sulfur dioxide. The slurry containing both gypsum and unreacted lime or limestone is then collected at the bottom of the chamber and recycled.
Typical wet scrubbing systems can capture up to 97% of the sulfur within the flue gas. With special additives, this can be raised to 99% in some cases. Wet scrubbers can easily be fitted to existing power plants, provided the space is available. Wet scrubbing technology is technically complex. It has been likened to a chemical plant operating within a power station. For this reason it requires skilled staff to operate. Nevertheless, it provides a proven method for removing high levels of the sulfur from a coal-fired power plant’s flue-gas stream.
A variation on the lime or limestone scrubbing system is the seawater FGD system. Instead of the calcium-based absorbents, this uses seawater pumped directly from the sea in the scrubbing tower to absorb sulfur dioxide. Seawater is naturally alkaline and will react with the sulfur dioxide, generating soluble sulfates that are carried away with the seawater and eventually released again into the sea. Seawater scrubbing can only be used when power plants are built on coastal sites but is claimed to be capable of 98–99% removal efficiency.
Other, more complex treatment systems have been tested including the use of activated carbon or charcoal. This is capable of absorbing both sulfur dioxide and nitrogen oxide and can eventually be regenerated, leaving pure sulfur as one by-product. Activated carbon can also absorb trace elements such as mercury.
Related posts:
Incoming search terms:
- how scrubbers remove sulphur dioxide from the gas stream emitted from power plants
- effectiveness of sulfur removing controls in coal-fired power plants when you compare the map of controls with the acid rain content in the region
- how can sulphur dioxide be removed from flue gases
- Sulfur Dioxide Removal by Seawater
- Sulfur in the Furnace
- sulfur remuval plant from jigs
- Sulpher Dioxide mail
- Wet Ammonia FGD (Flue Gas Desulfurization) for power plant mail